Superheavy Element Research Superheavy Element Research
Superheavy Element Research Superheavy Element Research
Superheavy Element Research Superheavy Element Research
You also want an ePaper? Increase the reach of your titles
YUMPU automatically turns print PDFs into web optimized ePapers that Google loves.
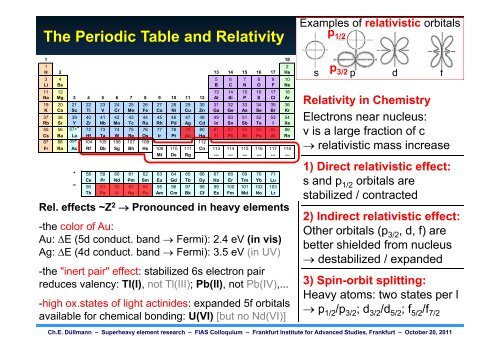
The Periodic Table and Relativity<br />
1 18<br />
1 2<br />
H 2 13 14 15 16 17 He<br />
3 4 5 6 7 8 9 10<br />
Li Be B C N O F Ne<br />
11 12 13 14 15 16 17 18<br />
NNa MMg 3 4 5 6 7 8 9 10 11 12 Al Si P S Cl AAr<br />
19 20 21 22 23 24 25 26 27 28 29 30 31 32 33 34 35 36<br />
K Ca Sc Ti V Cr Mn Fe Co Ni Cu Zn Ga Ge As Se Br Kr<br />
37 38 39 40 41 42 43 44 45 46 47 48 49 50 51 52 53 54<br />
Rb Sr Y Zr Nb Mo Tc Ru Rh Pd Ag Cd In Sn Sb Te I Xe<br />
55 56 57+* 72 73 74 75 76 77 78 79 80 81 82 83 84 85 86<br />
Cs<br />
87<br />
Ba<br />
88<br />
La<br />
89+"<br />
Hf<br />
104<br />
Ta<br />
105<br />
W<br />
106<br />
Re<br />
107<br />
Os<br />
108<br />
Ir Pt Au Hg<br />
112<br />
Tl Pb Bi Po At Rn<br />
Fr Ra Ac Rf Db Sg Bh Hs 109 110 111 Cn 113 114 115 116 117 118<br />
Mt Ds Rg --- --- --- --- --- ---<br />
Examples of relativistic orbitals<br />
p1/2 p 3/2<br />
s p d f<br />
RRelativity l ti it in i Chemistry Ch i t<br />
Electrons near nucleus:<br />
v is a large g fraction of c<br />
� relativistic mass increase<br />
1) Direct relativistic effect:<br />
*<br />
"<br />
58<br />
Ce<br />
90<br />
Th<br />
59<br />
Pr<br />
91<br />
Pa<br />
60<br />
Nd<br />
92<br />
U<br />
61<br />
Pm<br />
93<br />
Np<br />
62<br />
Sm<br />
94<br />
Pu<br />
63<br />
Eu<br />
95<br />
Am<br />
64<br />
Gd<br />
96<br />
Cm<br />
65<br />
Tb<br />
97<br />
Bk<br />
66<br />
Dy<br />
98<br />
Cf<br />
67<br />
Ho<br />
99<br />
Es<br />
68<br />
Er<br />
100<br />
Fm<br />
69<br />
Tm<br />
101<br />
Md<br />
70<br />
Yb<br />
102<br />
No<br />
71<br />
Lu<br />
103<br />
Lr<br />
s and d p1/2 orbitals bit l are<br />
stabilized / contracted<br />
Rel. effects ~Z<br />
2) Indirect relativistic effect:<br />
2 � Pronounced in heavy elements<br />
-the color of Au:<br />
Au: �E (5d conduct. band � Fermi): 2.4 eV (in vis)<br />
Ag: g �E (4d ( conduct. band � Fermi): ) 3.5 eV (in ( UV) )<br />
2) Indirect relativistic effect:<br />
Other orbitals (p3/2, d, f) are<br />
better shielded from nucleus<br />
� ddestabilized t bili d / expanded d d<br />
-the "inert pair" effect: stabilized 6s electron pair<br />
reduces valency: Tl(I), not Tl(III); Pb(II), not Pb(IV),...<br />
-high ox.states of light actinides: expanded 5f orbitals<br />
available for chemical bonding: U(VI) [but no Nd(VI)]<br />
3) Spin-orbit splitting:<br />
Heavy atoms: two states per l<br />
� p1/2/p3/2; d3/2/d5/2; f5/2/f7/2 Ch.E. Düllmann – <strong>Superheavy</strong> element research – FIAS Colloquium – Frankfurt Institute for Advanced Studies, Frankfurt – October 20, 2011