Superheavy Element Research Superheavy Element Research
Superheavy Element Research Superheavy Element Research
Superheavy Element Research Superheavy Element Research
Create successful ePaper yourself
Turn your PDF publications into a flip-book with our unique Google optimized e-Paper software.
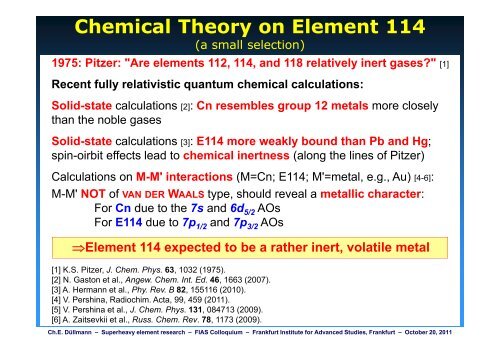
Chemical Theory on <strong>Element</strong> 114<br />
(a small selection)<br />
1975: Pitzer: "Are elements 112, 114, and 118 relatively inert gases?" [1]<br />
Recent fully relativistic quantum chemical calculations:<br />
Solid-state calculations [2]: Cn resembles group 12 metals more closely<br />
than the noble gases<br />
Solid-state calculations [3]: E114 more weakly bound than Pb and Hg;<br />
spin-oirbit effects lead to chemical inertness (along the lines of Pitzer)<br />
Calculations on MM-M M' interactions (M=Cn; E114; M'=metal M=metal, ee.g., g Au) [4 [4-6]: 6]:<br />
M-M' NOT of VAN DER WAALS type, should reveal a metallic character:<br />
For Cn due to the 7s and 6d 5/2 AOs<br />
For E114 due to 7p 1/2 and 7p 3/2 AOs<br />
�<strong>Element</strong> 114 expected to be a rather inert, volatile metal<br />
[1] K.S. Pitzer, J. Chem. Phys. 63, 1032 (1975).<br />
[2] N. Gaston et al., Angew. Chem. Int. Ed. 46, 1663 (2007).<br />
[3] A. Hermann et al., Phy. Rev. B 82, 155116 (2010).<br />
[4] V. Pershina, Radiochim. Acta, 99, 459 ( (2011). )<br />
[5] V. Pershina et al., J. Chem. Phys. 131, 084713 (2009).<br />
[6] A. Zaitsevkii et al., Russ. Chem. Rev. 78, 1173 (2009).<br />
Ch.E. Düllmann – <strong>Superheavy</strong> element research – FIAS Colloquium – Frankfurt Institute for Advanced Studies, Frankfurt – October 20, 2011