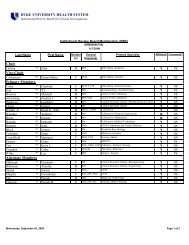
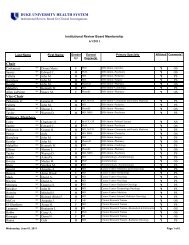
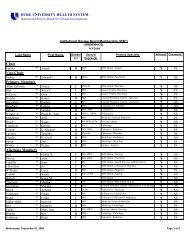
The Principal Investigator's Responsibilities in Research
The Principal Investigator's Responsibilities in Research
The Principal Investigator's Responsibilities in Research
You also want an ePaper? Increase the reach of your titles
YUMPU automatically turns print PDFs into web optimized ePapers that Google loves.
<strong>The</strong> <strong>Pr<strong>in</strong>cipal</strong> Investigator’s<br />
<strong>Responsibilities</strong> <strong>in</strong> <strong>Research</strong><br />
Morn<strong>in</strong>g Presenter: David Rizzieri, MD<br />
Associate Professor, Cancer Center<br />
Afternoon Presenter: Edward Suarez, PhD<br />
Associate Professor, Department of Psychiatry
David Rizzieri, MD<br />
Associate Professor, Cancer Center<br />
Dr. Rizzieri is an Associate Professor of Medic<strong>in</strong>e and <strong>in</strong>volved <strong>in</strong><br />
the hematologic malignancy program, writ<strong>in</strong>g and conduct<strong>in</strong>g<br />
over 50 studies of novel agents <strong>in</strong> the treatment of leukemia and<br />
lymphoma. In addition, he is funded as a 'Cl<strong>in</strong>ical Scholar of the the<br />
Leukemia and Lymphoma Society' for his work us<strong>in</strong>g<br />
mismatched immune systems <strong>in</strong> hematopoietic stem cell<br />
transplantation and <strong>in</strong>troduc<strong>in</strong>g new post transplant immune<br />
modulatory<br />
strategies.
Duke University Health System<br />
PI <strong>Responsibilities</strong> & <strong>The</strong> IRB<br />
Duke Policy states that ALL human subjects’<br />
research done at Duke or done by Duke faculty,<br />
staff or students anywhere <strong>in</strong> the world MUST<br />
have DUHS IRB review and approval prior to<br />
<strong>in</strong>itial human subject contact.
DUHS PRINCIPAL<br />
INVESTIGATOR (PI)<br />
AGREEMENT<br />
New DUHS PI Agreement of 22 Statements<br />
To Be Signed Annually by <strong>Research</strong>ers<br />
Implementation date to be announced by Dr.<br />
Oddone
DUHS PI Agreement<br />
Commitment of a DUHS PI or Co-PI Co PI to<br />
Institutional Human Subject Protection<br />
Policies & IRB Oversight Under Federal- Federal<br />
Wide Assurance, FWA # 00009025
DUHS PI Agreement<br />
Preamble<br />
Declaration of <strong>in</strong>tent to participate <strong>in</strong><br />
research subject to <strong>in</strong>itial and cont<strong>in</strong>u<strong>in</strong>g review<br />
by the IRB.
DUHS PI Agreement<br />
Preamble cont<strong>in</strong>ued<br />
Understand<strong>in</strong>g that a condition of do<strong>in</strong>g<br />
research is to acknowledge and accept<br />
responsibilities of the DUHS Investigator<br />
Agreement to comply with <strong>in</strong>stitutional policies,<br />
applicable federal, state, & local laws and<br />
regulations, ethical guidel<strong>in</strong>es and other policies<br />
and pr<strong>in</strong>ciples as described <strong>in</strong> the document.
1. Familiar with:<br />
-HHS 45 CFR 46:<br />
DUHS PI Agreement<br />
-FDA 21 CFR 50 & 56:<br />
54, 312, 314:<br />
601:<br />
812:<br />
814:<br />
Protection of Human Subjects<br />
Protection of Human Subjects<br />
Good Cl<strong>in</strong>ical Practice<br />
Licens<strong>in</strong>g of Biologics<br />
Investigational Device Exemptions<br />
Pre-market Approval of Medical Devices<br />
-HIPAA 45 CFR 46, 160 and 164
DUHS PI <strong>Responsibilities</strong><br />
2. I recognize the authority of the DUHS<br />
IRB<br />
oversee human subject research, as<br />
described <strong>in</strong> the FWA, and I will abide by<br />
all decisions of the IRB
DUHS PI <strong>Responsibilities</strong><br />
3. Assume overall adm<strong>in</strong>istrative responsibilities<br />
for all aspects<br />
of each protocol approved under<br />
this Agreement. Will ma<strong>in</strong>ta<strong>in</strong> appropriate<br />
oversight<br />
of the research protocol and my<br />
research staff and appropriately delegate<br />
research responsibilities.
4.<br />
DUHS PI <strong>Responsibilities</strong><br />
I will ensure that all members of my research staff, and<br />
all others directly <strong>in</strong>volved <strong>in</strong> the conduct of the<br />
study, are qualified by education, tra<strong>in</strong><strong>in</strong>g, and<br />
experience to perform their research<br />
responsibilities.<br />
I will <strong>in</strong>form my staff of any pert<strong>in</strong>ent changes dur<strong>in</strong>g the<br />
course of a study, and arrange for education or<br />
additional tra<strong>in</strong><strong>in</strong>g of staff as needed.
DUHS PI <strong>Responsibilities</strong><br />
5. If I arrange with a source outside of DUHS to<br />
provide <strong>in</strong>formation critical to the study, I will<br />
take steps to ensure that the outside source can<br />
verify the <strong>in</strong>tegrity of data and records<br />
provided to me.
DUHS PI <strong>Responsibilities</strong><br />
6. Will employ sound study design<br />
<strong>in</strong> accordance<br />
with the standards of my discipl<strong>in</strong>e.
DUHS PI <strong>Responsibilities</strong><br />
7. Will recruit participants <strong>in</strong> a fair and equitable<br />
manner, manner,<br />
weigh<strong>in</strong>g the potential benefits of the<br />
research to the participants aga<strong>in</strong>st their<br />
vulnerability and the risks to them.
DUHS PI <strong>Responsibilities</strong><br />
8. I will notify the IRB when I wish to enroll a<br />
prospective subject who does not meet<br />
<strong>in</strong>clusion/exclusion criteria as specified <strong>in</strong> the<br />
protocol, and I will do so prior to enrollment<br />
of the person.
DUHS PI <strong>Responsibilities</strong><br />
9. For more than m<strong>in</strong>imal risk research will<br />
provide the IRB with an adequate data and<br />
safety monitor<strong>in</strong>g plan for promptly detect<strong>in</strong>g<br />
harm and mitigat<strong>in</strong>g potential <strong>in</strong>juries.
DUHS PI <strong>Responsibilities</strong><br />
10. I have determ<strong>in</strong>ed, before conduct<strong>in</strong>g the<br />
research study, that the resources necessary to<br />
appropriately protect participants are present.
DUHS PI <strong>Responsibilities</strong><br />
11. I will comply with the IRB’s prompt report<strong>in</strong>g<br />
requirements.
DUHS PI <strong>Responsibilities</strong><br />
12. Will seek, document, and ma<strong>in</strong>ta<strong>in</strong> records of<br />
<strong>in</strong>formed consent and HIPAA authorization<br />
from<br />
each research participant or the participant’s legally<br />
authorized representative as required under HHS<br />
regulations and stipulated by the IRB.<br />
Will<br />
develop an <strong>in</strong>formed consent process<br />
emphasiz<strong>in</strong>g the importance of participant<br />
comprehension and voluntary participation.
DUHS PI <strong>Responsibilities</strong><br />
13. I will ensure that the <strong>in</strong>formed consent process is led<br />
only by <strong>in</strong>dividuals who have appropriate tra<strong>in</strong><strong>in</strong>g and<br />
knowledge of the research, <strong>in</strong>clud<strong>in</strong>g any<br />
<strong>in</strong>vestigational product <strong>in</strong>volved, <strong>in</strong> order to discuss<br />
the risks and benefits of the study with prospective<br />
subjects.<br />
Only appropriate staff listed as “Key Personnel” <strong>in</strong><br />
my IRB submission will be authorized by me to<br />
conduct the consent process with prospective<br />
subjects.
DUHS PI <strong>Responsibilities</strong><br />
14. I agree to cooperate with the IRB as it conducts <strong>in</strong>itial<br />
and cont<strong>in</strong>u<strong>in</strong>g review, <strong>in</strong>clud<strong>in</strong>g provid<strong>in</strong>g required<br />
<strong>in</strong>formation, records, reports, and certifications.<br />
I will ensure that the periodic cont<strong>in</strong>u<strong>in</strong>g review of my<br />
research will occur with<strong>in</strong> the time frame stipulated<br />
by the IRB, and no research will cont<strong>in</strong>ue beyond the<br />
designated approval period.
DUHS PI <strong>Responsibilities</strong><br />
15. Will conduct research <strong>in</strong>volv<strong>in</strong>g FDA-regulated FDA regulated products<br />
under an IND application or IDE,<br />
Will comply with all applicable FDA regulations and fulfill<br />
all <strong>in</strong>vestigator responsibilities [or <strong>in</strong>vestigator-sponsor<br />
<strong>in</strong>vestigator sponsor<br />
responsibilities, where appropriate], <strong>in</strong>clud<strong>in</strong>g those<br />
described at 21 CFR 312 and 812, the text of which is<br />
found at:<br />
http://www.accessdata.fda.gov/scripts/cdrh/cfdocs/cfcfr<br />
/cfrsearch.cfm
DUHS PI <strong>Responsibilities</strong><br />
16. I Will NOT enroll participants <strong>in</strong> research prior to the review<br />
and approval of the research by the IRB.<br />
I Will report promptly to the IRB proposed changes <strong>in</strong> the<br />
research conducted under this Agreement.<br />
I Will NOT <strong>in</strong>itiate changes <strong>in</strong> research activities without<br />
prior review and approval by the IRB, except when necessary<br />
to elim<strong>in</strong>ate immediate hazards to the research participants.
DUHS PI <strong>Responsibilities</strong><br />
17. Understand that emergency medical care may<br />
be delivered without IRB review and approval<br />
to the extent permitted under applicable<br />
Federal regulations and State law.
DUHS PI <strong>Responsibilities</strong><br />
18. I (and my research staff) will respond <strong>in</strong> a<br />
timely manner to participants’ compla<strong>in</strong>ts or<br />
requests for <strong>in</strong>formation.<br />
If I am unable to resolve the compla<strong>in</strong>t<br />
satisfactorily, then it is my responsibility to<br />
report the compla<strong>in</strong>t to the IRB.
DUHS PI <strong>Responsibilities</strong><br />
19. I will generally be available (by phone or other<br />
electronic communication) to subjects dur<strong>in</strong>g the<br />
study. If I will be unavailable dur<strong>in</strong>g the study, I will<br />
delegate study responsibility to a specific qualified<br />
person who will be available <strong>in</strong> my absence. I will<br />
<strong>in</strong>form the IRB of this delegation of authority, via a<br />
protocol amendment, as a change <strong>in</strong> the research<br />
activity requir<strong>in</strong>g IRB review.
DUHS PI <strong>Responsibilities</strong><br />
20. Understand if there is a change <strong>in</strong> my status<br />
as PI, it is my responsibility to <strong>in</strong>form the IRB<br />
of the change & to seek IRB approval for a<br />
new pr<strong>in</strong>cipal <strong>in</strong>vestigator to cont<strong>in</strong>ue the<br />
project,<br />
or to request closure of the protocol.
DUHS PI <strong>Responsibilities</strong><br />
21. I will cooperate with any <strong>in</strong>quiry by the Duke<br />
University School of Medic<strong>in</strong>e Compliance Office<br />
concern<strong>in</strong>g any research with humans <strong>in</strong> which I<br />
participate.<br />
In the event that <strong>in</strong>stitutional officials determ<strong>in</strong>e that<br />
I have failed to comply with this Agreement, I agree<br />
to take recommended action (s), <strong>in</strong>clud<strong>in</strong>g but not<br />
limited to term<strong>in</strong>ation of my participation <strong>in</strong><br />
designated research activities.
DUHS PI <strong>Responsibilities</strong><br />
22. In the event I am found to have failed to comply of<br />
with any of these requirements, the IRB will report<br />
such noncompliance to <strong>in</strong>stitutional officials, the<br />
Office of Human <strong>Research</strong> Protections (OHRP), the<br />
compliance officer of any other sponsor<strong>in</strong>g federal<br />
department or agency, such as the FDA, and the non-<br />
federal sponsor of the research, as appropriate.
DUHS Investigator Agreement &<br />
Responsibility<br />
23. I acknowledge that my primary responsibility as a<br />
PI or Co-PI Co PI is to safeguard the rights and welfare of<br />
each research subject, and that the subject’s rights and<br />
welfare must take precedence over the goals and<br />
requirements of the research.





![Human Research Protection Program [Dr. Wesley Byerly]](https://img.yumpu.com/50293157/1/190x143/human-research-protection-program-dr-wesley-byerly.jpg?quality=85)