Thermodynamics - Apple
Thermodynamics - Apple
Thermodynamics - Apple
Create successful ePaper yourself
Turn your PDF publications into a flip-book with our unique Google optimized e-Paper software.
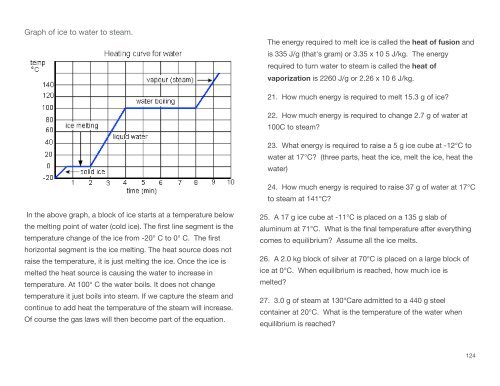
Graph of ice to water to steam.<br />
In the above graph, a block of ice starts at a temperature below<br />
the melting point of water (cold ice). The first line segment is the<br />
temperature change of the ice from -20° C to 0° C. The first<br />
horizontal segment is the ice melting. The heat source does not<br />
raise the temperature, it is just melting the ice. Once the ice is<br />
melted the heat source is causing the water to increase in<br />
temperature. At 100° C the water boils. It does not change<br />
temperature it just boils into steam. If we capture the steam and<br />
continue to add heat the temperature of the steam will increase.<br />
Of course the gas laws will then become part of the equation.<br />
The energy required to melt ice is called the heat of fusion and<br />
is 335 J/g (that's gram) or 3.35 x 10 5 J/kg. The energy<br />
required to turn water to steam is called the heat of<br />
vaporization is 2260 J/g or 2.26 x 10 6 J/kg.<br />
21. How much energy is required to melt 15.3 g of ice?<br />
22. How much energy is required to change 2.7 g of water at<br />
100C to steam?<br />
23. What energy is required to raise a 5 g ice cube at -12°C to<br />
water at 17°C? (three parts, heat the ice, melt the ice, heat the<br />
water)<br />
24. How much energy is required to raise 37 g of water at 17°C<br />
to steam at 141°C?<br />
25. A 17 g ice cube at -11°C is placed on a 135 g slab of<br />
aluminum at 71°C. What is the final temperature after everything<br />
comes to equilibrium? Assume all the ice melts.<br />
26. A 2.0 kg block of silver at 70°C is placed on a large block of<br />
ice at 0°C. When equilibrium is reached, how much ice is<br />
melted?<br />
27. 3.0 g of steam at 130°Care admitted to a 440 g steel<br />
container at 20°C. What is the temperature of the water when<br />
equilibrium is reached?<br />
124