dry anaerobic digestion of municipal solid waste and digestate ...
dry anaerobic digestion of municipal solid waste and digestate ...
dry anaerobic digestion of municipal solid waste and digestate ...
You also want an ePaper? Increase the reach of your titles
YUMPU automatically turns print PDFs into web optimized ePapers that Google loves.
2.3 Inhibition <strong>of</strong> Dry Anaerobic Digestion<br />
Dry <strong>anaerobic</strong> <strong>digestion</strong> <strong>of</strong> organic <strong>solid</strong> <strong>waste</strong> faces many inhibition problems which are<br />
harder to control (Guendouz et al., 2010). These include inhibition by volatile fatty acids<br />
(VFA), ammonia, heavy metals <strong>and</strong> metals, as given in Table 2.1 with their inhibitory<br />
concentrations. The main inhibitors <strong>of</strong> <strong>dry</strong> <strong>anaerobic</strong> <strong>digestion</strong> process (ammonia <strong>and</strong><br />
VFA) have been discussed in detail as under.<br />
2.3.1 Volatile fatty acids (VFA)<br />
Methanogens are susceptible to high concentrations <strong>of</strong> acids in reactor, so acid conditions<br />
can inhibit their growth. Volatile fatty acids are intermediate compounds <strong>of</strong> methanation<br />
<strong>and</strong> their high concentration can cause stress to microbes. The mainly produced<br />
intermediates during <strong>anaerobic</strong> <strong>digestion</strong> <strong>of</strong> organics are acetic, propionic, butyric <strong>and</strong><br />
valeric acids (Buyukkamaci <strong>and</strong> Filibeli, 2004) whereas the concentration <strong>of</strong> acetic <strong>and</strong><br />
propionic acid is a useful measure for performance <strong>of</strong> digester.<br />
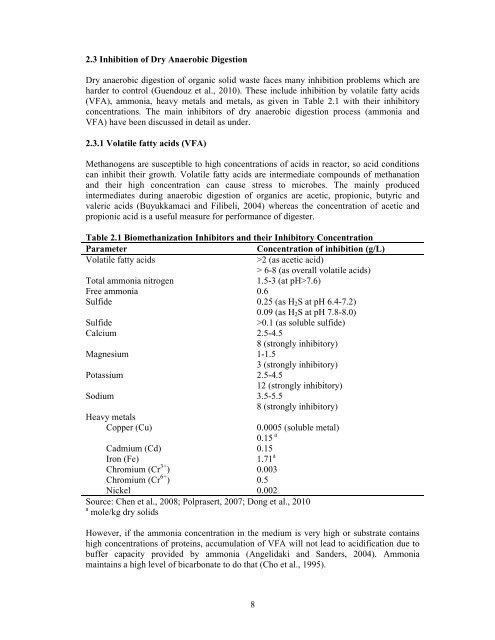
Table 2.1 Biomethanization Inhibitors <strong>and</strong> their Inhibitory Concentration<br />
Parameter Concentration <strong>of</strong> inhibition (g/L)<br />
Volatile fatty acids >2 (as acetic acid)<br />
> 6-8 (as overall volatile acids)<br />
Total ammonia nitrogen 1.5-3 (at pH>7.6)<br />
Free ammonia 0.6<br />
Sulfide 0.25 (as H2S at pH 6.4-7.2)<br />
0.09 (as H2S at pH 7.8-8.0)<br />
Sulfide >0.1 (as soluble sulfide)<br />
Calcium 2.5-4.5<br />
8 (strongly inhibitory)<br />
Magnesium 1-1.5<br />
3 (strongly inhibitory)<br />
Potassium 2.5-4.5<br />
12 (strongly inhibitory)<br />
Sodium 3.5-5.5<br />
8 (strongly inhibitory)<br />
Heavy metals<br />
Copper (Cu)<br />
Cadmium (Cd)<br />
Iron (Fe)<br />
Chromium (Cr 3+ )<br />
Chromium (Cr 6+ )<br />
Nickel<br />
8<br />
0.0005 (soluble metal)<br />
0.15 a<br />
0.15<br />
1.71 a<br />
0.003<br />
0.5<br />
0.002<br />
Source: Chen et al., 2008; Polprasert, 2007; Dong et al., 2010<br />
a mole/kg <strong>dry</strong> <strong>solid</strong>s<br />
However, if the ammonia concentration in the medium is very high or substrate contains<br />
high concentrations <strong>of</strong> proteins, accumulation <strong>of</strong> VFA will not lead to acidification due to<br />
buffer capacity provided by ammonia (Angelidak i <strong>and</strong> S<strong>and</strong>ers, 2004). Ammonia<br />
maintains a high level <strong>of</strong> bicarbonate to do that (Cho et al., 1995).