Lecture 12: Crystallization & Mineral Reactions Read Chpt 2
Lecture 12: Crystallization & Mineral Reactions Read Chpt 2
Lecture 12: Crystallization & Mineral Reactions Read Chpt 2
Create successful ePaper yourself
Turn your PDF publications into a flip-book with our unique Google optimized e-Paper software.
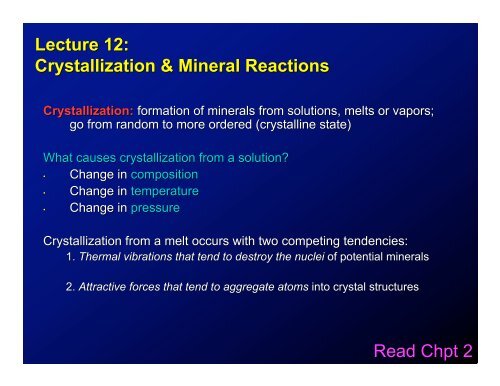
<strong>Lecture</strong> <strong>12</strong>:<br />
<strong>Crystallization</strong> & <strong>Mineral</strong> <strong>Reactions</strong><br />
<strong>Crystallization</strong>: formation of minerals from solutions, melts or vapors;<br />
go from random to more ordered (crystalline state)<br />
What causes crystallization from a solution?<br />
• Change in composition<br />
• Change in temperature<br />
• Change in pressure<br />
<strong>Crystallization</strong> from a melt occurs with two competing tendencies:<br />
1. Thermal vibrations that tend to destroy the nuclei of potential minerals<br />
2. Attractive forces that tend to aggregate atoms into crystal structures<br />
<strong>Read</strong> <strong>Chpt</strong> 2
Crystal Growth: : How do they form??<br />
Nucleation: a “seed” crystal forms (aka(<br />
“nucleus”); ions constantly come<br />
together, but most redissolve. . Why? Atoms at the surface have<br />
unsatistified bonds (incomplete polyhedra). More surface atoms per unit<br />
solid = less stability (high surface energy)<br />
Critical Size: : must be reached to grow a crystal; if seed grows rapidly, it<br />
will reach a point where the surface energy is lowered enough that<br />
crystallite can keep growing and not be redissolved; ; ions stick best at<br />
steps and kinks, where they can satisfy more unsatisfied bonds at once
MINERAL REACTIONS<br />
Igneous <strong>Reactions</strong>:<br />
95% of the earth’s s crust is composed of igneous rocks that form from<br />
magmas<br />
Magmas are principally composed of O, Si, , Al, Fe, Ca, Mg, Na and K<br />
(with accessory H 2 O, CO 2 and volatiles like H 2 S, HCl, , CH 4 , CO)<br />
As magmas cool, minerals begin to precipitate in a distinct order:<br />
‘Bowen’s s Reaction Series’
Bowen wanted to answer the question:<br />
How do different types of igneous rocks form? Can you get basalts and<br />
granites from the same “parent” magma?<br />
So, he did a series of lab experiments:<br />
1. Make artificial magma of ‘basaltic’ composition<br />
2. Cool very slowly to a particular temperature<br />
3. Quickly quench<br />
Results? Two different types of “reaction series”<br />
Continuous Reaction Series: : a solid solution of continuously changing<br />
composition forms<br />
Ex. Plagioclase feldspars (Ca rich => Na rich at lower T)<br />
Discontinuous Reaction Series: reactions occur between melt and<br />
previously precipitated crystals; the old crystals dissolve and new<br />
minerals of different composition and structure form<br />
Ex. Olivine => Pyroxene => Amphibole => Biotite
Bowen’s s idea:<br />
Magmatic Differentiation: a single<br />
single homogeneous magma<br />
produces a variety of chemically distinct igneous rocks because of…<br />
Frractional <strong>Crystallization</strong>: crystals are<br />
crystals are physically separated from<br />
the cooling magma (e.g., by gravity settling or rimming) so that liquid and<br />
crystal cannot react further; ; changes the bulk composition of the magma<br />
(in fact, makes it more SiO 2 -rich, because mafic minerals form first and<br />
settle out)<br />
We will discuss this more in the context of phase diagrams…Bowen was<br />
partially correct, but there are other processes at work as well…
MINERAL REACTIONS<br />
Metamorphic <strong>Reactions</strong>:<br />
Reaction that occur in the solid state<br />
-typically isochemical, , that is, the bulk chemistry is unchanged (except often<br />
a change in H 2 O)<br />
Examples:<br />
Dehydration reactions: higher T, volatile H 2 O is formed and lost<br />
Decarbonation reactions: : carbonate-rich sedimentary rocks lose<br />
CO 2 with increasing T<br />
CaCO 3 (calcite) + SiO 2 (silica) = CaSiO 3 (wollastonite)) + CO 2 (gas)<br />
Metasomatic: : additional elements are gained or lost by circulating fluids
MINERAL REACTIONS<br />
Weathering <strong>Reactions</strong> (Physical vs. . Chemical):<br />
Chemical Weathering: thermodynamically driven alteration of minerals to<br />
form new minerals; changes composition and structure; occurs because of<br />
changes in T, P, composition<br />
Primary <strong>Mineral</strong>s: : Quartz, Olivine, Albite, Enstatite, , Muscovite<br />
tend to weather and form<br />
Secondary <strong>Mineral</strong>s: Kaolinite, , Goethite, Gibbsite, Hematite<br />
“Goldich” Weathering Series: : susceptibility to chemical weathering<br />
Quartz
MINERAL REACTIONS<br />
“Goldich” Weathering Series:<br />
Quartz
So what is the answer?<br />
Differences in structure, bonding determine weathering kinetics<br />
More polymerized structures break apart more slowly (more strong bonds<br />
to break!)<br />
Sometimes the reaction product is not solid phase, e.g. secondary layer<br />
silicates, but rather dissolved SiO 2 , Na + , K + , Ca +2 , Mg +2<br />
Examples:<br />
Al 2 SiO 5 (sillimanite)) + SiO 2 (quartz) + H 2 O => Al 2 Si 2 O 5 (OH) 4 (kaolinite)<br />
3KAlSi 3 O 8 (orthoclase) + 2H + => KAl 3 Si 3 O 10 (OH) 2 (muscovite) + 2K + + 6SiO 2
MINERAL REACTIONS<br />
Ultra High Pressure <strong>Reactions</strong>:<br />
Below the crust at high temperatures and pressures, minerals undergo<br />
transformation reactions into structures more suited to those P,T<br />
conditions<br />
How do we know?<br />
Specimens at the surface, e.g., from kimberlite pipes<br />
Laboratory experiments with diamond anvil cells<br />
* Below 6km, Si probably exists in 6-fold coordination with O, rather than<br />
4-fold<br />
* Increased densities of materials, denser packing of elements<br />
* Perovskite and rutile type structures become important*
Rutile Structure (TiO 2 )<br />
Based on hexagonal closest packing (HCP): ABABABAB layers of HP<br />
Ti atoms fill half of the possible octahedral positions<br />
The cations are always in 6-fold (octahedral) coordination.<br />
The anions are coordinated by 3 cations (trigonal<br />
coordination)<br />
Octahedra link along edges
Perovskite Structure (ABO 3 )<br />
Cubic closest packing (CCP) of oxygen, e.g., ABCABC layers of HP<br />
1/4 of oxygens are replaced by a large A cation<br />
A cation is in <strong>12</strong>-fold coordination with surrounding O<br />
B cation is in 6-fold (octahedral) coordination with O<br />
Octahedra share apices only<br />
Very dense structure