Supplier Registration Form - DVED
Supplier Registration Form - DVED
Supplier Registration Form - DVED
Create successful ePaper yourself
Turn your PDF publications into a flip-book with our unique Google optimized e-Paper software.
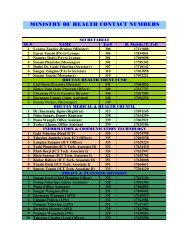
ROYAL GOVERNMENT OF BHUTAN<br />
MINISTRY OF HEALTH<br />
DEPARTMENT OF MEDICAL SERVICES<br />
DRUGS VACCINE AND EQUIPMENT DIVISION<br />
APLICATION FOR REGISTRATION AS SUPPLIER OF DRUGS/NONDRUGS ONTO THE <strong>DVED</strong><br />
SUPPLIER REGISTER<br />
Name of the company: _____________________________________________________<br />
Applicant’s name: _________________________________________________________<br />
Contact Person: ________________________________________________________________________________<br />
Contact Person’s Tel no: ______________________________________________________________________<br />
For use by <strong>DVED</strong> Officials only<br />
Date of submission:...............................................................................................................................................<br />
Received by:………………………………………………………................................................................................<br />
1
ROYAL GOVERNMENT OF BHUTAN<br />
MINISTRY OF HEALTH<br />
DEPARTMENT OF MEDICAL SERVICES<br />
DRUGS VACCINE AND EQUIPMENT DIVISION<br />
1. PURPOSE<br />
Terms of Reference for <strong>Registration</strong> onto <strong>DVED</strong> <strong>Supplier</strong>s Register<br />
1.1. The <strong>DVED</strong> shall maintain a <strong>Supplier</strong> Register containing detailed information on all suppliers registered<br />
with the division for the supply of drugs and non‐drugs. This is being done to ensure that there are<br />
sufficient qualified suppliers for the supply of drugs/non drugs and medical items/services of ensured<br />
quality as and when required.<br />
1.2. Maintenance of the <strong>Supplier</strong>s Register: The <strong>DVED</strong> will update suppliers’ information on an ongoing<br />
basis. Registered suppliers shall be responsible for providing <strong>DVED</strong> with any change in the information<br />
initially provided, including banking details. Failing to do so, <strong>DVED</strong> reserves the right to cancel the<br />
registration. It is the supplier’s responsibility to ensure that the information reflected on the <strong>Supplier</strong>s<br />
Register is correct and up to date at all times.<br />
2. CRIT ERIA FOR REGISTRATION<br />
2.1 all suppliers who wish to be registered onto the <strong>Supplier</strong> Register must meet the set criteria, as<br />
specified below:<br />
• Valid Physical Address<br />
• Contact details such as physical Business Address, postal Address, telephone or cell number,<br />
fax number<br />
• Company/Business Profile<br />
• A line of products intended to supply or specialized in.<br />
• Banking details. Personal banking details will not be acceptable except in the case where the<br />
supplier is a Sole Trader.<br />
• Bank Guarantee from any recognized financial institute in Bhutan or in the country of origin<br />
• Proof of financial soundness.<br />
• Tax Clearance Certificate<br />
• CID copies of all proprietors or partners ( where applicable)<br />
• Trade licence issued by the Ministry of Economics and Affairs, Royal Government of Bhutan.<br />
For non‐national suppliers, a valid trade licence issued by relevant authority in their country<br />
where the business is registered should be submitted.<br />
• Manufacturing license<br />
• WHO GMP certificates in case where the supplier manufacturers any products such as<br />
pharmaceuticals and laboratory re‐agents etc.<br />
Note: all the above documents should be valid at the time of submission and during the validity period of<br />
registration. A physical inspection by a team shall also be carried out at site if deemed necessary.<br />
3. HOW TO REGISTER<br />
3.1 Any supplier who wishes to register onto the <strong>DVED</strong> <strong>Supplier</strong>s Register should complete the<br />
following:<br />
a) The Application <strong>Form</strong> Application for <strong>Registration</strong> as a <strong>Supplier</strong> of drugs, vaccines, medical<br />
supplies and Services onto the <strong>Supplier</strong>s Register<br />
b) If a firm has more than one branch office and would like to register them all, separate application<br />
forms must be filled for each branch<br />
NB‐ Please uses a black pen. Please print so that all information is legible. <strong>Form</strong>s which are not<br />
readable or incomplete will be rejected<br />
3.2 Availability of Application <strong>Form</strong>s: Application <strong>Form</strong>s can be downloaded from MOH website<br />
(http://www.health.gov.bt). Application forms can also be collected from the <strong>DVED</strong>. No faxed or e‐<br />
2
ROYAL GOVERNMENT OF BHUTAN<br />
MINISTRY OF HEALTH<br />
DEPARTMENT OF MEDICAL SERVICES<br />
DRUGS VACCINE AND EQUIPMENT DIVISION<br />
mailed applications will be accepted. Only original and signed copies of application will be accepted.<br />
<strong>Supplier</strong>s may not alter the Application <strong>Form</strong> in any way.<br />
3.3 The applications forms for registration onto the <strong>Supplier</strong>s Register shall be processed at <strong>DVED</strong>.<br />
<strong>Supplier</strong>s should therefore ensure that they submit their Application <strong>Form</strong>s to the address below:<br />
DRUGS VACCINE AND EQUIPMENT DIVISION<br />
MINISTRY OF HEALTH<br />
THIMPHU BHUTAN<br />
PO BOX 985 TELEPHONE: 00975325458/325955/325956/326217<br />
Note: Please keep copies of the Application form and all supporting documentation submitted as no copies<br />
will be made by <strong>DVED</strong><br />
3.4 Any queries regarding registration can be directed to: <strong>DVED</strong> TELEPHONE: 00975‐<br />
325458/325955/325956/326217 FAX 323809<br />
EMAIL: dved@health.gov.bt or cpodved@health.gov.bt<br />
3.5 Ensure that all applicable sections in the Application <strong>Form</strong> are complete. Incomplete Application<br />
<strong>Form</strong>s will not be processed by <strong>DVED</strong>. Verification of information provided by suppliers may be<br />
done against third party sources such as financial institutes, relevant authorities such as embassies<br />
or physically.<br />
3.6 Important fields to be completed:<br />
3.6.1 Contact Person: Please provide details of one (1) individual that the <strong>DVED</strong> should<br />
contact pertaining to Bids and/or Contract and/or clarification and/or supply followup.<br />
3.6.2 Type of Company: Ensure the appropriate documentary proof pertaining to your type<br />
of Company is attached and submitted together with the Application <strong>Form</strong>.<br />
View below for the required documentary proof:<br />
a) Partnership: Certified copy of Partnership Agreement<br />
b) Sole Proprietor: CID copy<br />
c) Public Company: Certified copy of Certificate of Incorporation of companies<br />
d) Close Corporation.<br />
e) Private Company: Certified copy of Certificate of Incorporation of companies<br />
f) Trust: Certified copy of Trust deed or other founding document<br />
g) Other: Please provide appropriate documentary proo f<br />
3.7 Shareholder/Owner/partners Information please complete all information for every<br />
shareholder/Owner listed on the form who has equal ownership in the Company. Please ensure<br />
that Total percentage of ownership amount to 100%. Should the space provided in page No. 7<br />
(<strong>Form</strong> III) be inadequate for the required information, please ensure that you make a copy of form,<br />
complete it and submit it together with the Application <strong>Form</strong>.<br />
3.8 Applicants will be notified about the outcome of the application within 30 working days from the<br />
date of submission of forms to <strong>DVED</strong>, unless otherwise notified in advance by the division.<br />
3.9 A non‐refundable registration fee of Nu. 1,000/‐ (one thousand only) shall be charged to all the<br />
applicants at the time of submission of application.<br />
3
4. REGISTRATION VALIDITY<br />
ROYAL GOVERNMENT OF BHUTAN<br />
MINISTRY OF HEALTH<br />
DEPARTMENT OF MEDICAL SERVICES<br />
DRUGS VACCINE AND EQUIPMENT DIVISION<br />
4.1 <strong>Supplier</strong>s that have been registered onto the <strong>Supplier</strong>s Register shall be eligible to participate in<br />
the <strong>DVED</strong> annual tender for the supply of drugs and non‐drugs. <strong>Registration</strong> onto the <strong>Supplier</strong>s<br />
Register however does not guarantee business opportunities as all acquisition will be subject to the<br />
Procurement Manual of Bhutan.<br />
4.2 <strong>Registration</strong> Period onto the <strong>Supplier</strong>s Register: the validity of the <strong>Registration</strong> shall be for a<br />
period of 3 years from the date of acceptance onto the suppliers register.<br />
The Division reserves the right to accept or reject any application<br />
5. <strong>Supplier</strong> Performance Evaluation<br />
5.1 All registered suppliers shall be continuously monitored and evaluated on their performance based<br />
on the work awarded to them by <strong>DVED</strong> as per the guideline. This shall form the basis for annual<br />
suppliers’ performance evaluation which will have an impact on future registration and work<br />
opportunities with the <strong>DVED</strong>.<br />
5.2 The overall summary of the suppliers’ performance evaluation is presented to the Annual Tender<br />
Selection Committee meeting and those who have performed well in the past year may be given due<br />
preference during selection, where applicable/feasible.<br />
5.3 The supplier has to attain a minimum of 75% in their yearly performance depending on the orders<br />
received. Failing to do so, they will not be eligible to participate in the following year’s tender.<br />
<strong>Supplier</strong>’s who score more than 75% are further ranked as per the following:<br />
75 – 80% Satisfactory<br />
80 – 85% Good<br />
85 – 95% Very good<br />
> 95% Excellent<br />
An example of how the evaluation is done is shown below:<br />
<strong>Supplier</strong> name: X<br />
Number of items ordered: 10<br />
CRITERIA PERFORMANCE SCORE SCORE (in term s of %)<br />
Quantity supplied in full *<br />
Delivery of supply<br />
Supplied 9 out of 10 items in<br />
full<br />
8 out of 10 items supplied<br />
within the given deadline<br />
Quality of supply 4 items rejected during<br />
physical QC inspection. Had to<br />
be replaced<br />
9 / 10 90%<br />
8 / 10 80%<br />
6 / 10 60%<br />
Average Score 83.33%<br />
* Note: quantity supplied in full is defined as quantity > 90% of the ordered quantity. E.g. if 100,000 tablets of Drug<br />
A is ordered, and the supplier is able to deliver 95,000 tabs (95%), then the score given is 1. However, if the supplied<br />
quantity is 85,000 tabs (85%) of the ordered quantity, then the score is 0.<br />
Special considerations:<br />
For non‐drugs, the items are categorized into 16 departments such as laboratory, OT, dental etc., and therefore<br />
tender is also sold department wise. In this case, a supplier’s performance shall be rated department wise. So<br />
supplier X gets 60% for his performance in Dental Department and 80% for Anesthetic Department, the<br />
supplier shall be barred from participating in tender for Dental Department only.<br />
4
ROYAL GOVERNMENT OF BHUTAN<br />
MINISTRY OF HEALTH<br />
DEPARTMENT OF MEDICAL SERVICES<br />
DRUGS VACCINE AND EQUIPMENT DIVISION<br />
6.<br />
Confidentiality<br />
All information provided by suppliers for registration purposes shall remain confidential and shall be<br />
used by <strong>DVED</strong> for official purposes only unless otherwise required by law.<br />
5
ROYAL GOVERNMENT OF BHUTAN<br />
MINISTRY OF HEALTH<br />
DEPARTMENT OF MEDICAL SERVICES<br />
DRUGS VACCINE AND EQUIPMENT DIVISION<br />
I. COMPANY’S BASE DATA: (Compulsory)<br />
1 Name of Company:<br />
2 Business license from trade number<br />
( attach copy)<br />
3 TYPE OF COMPANY( Tick applicable box<br />
and provide documentary proof):<br />
O<br />
O<br />
O<br />
O<br />
O<br />
O<br />
O<br />
Close corporation<br />
Public Company<br />
One person business / sole trader<br />
Partnership<br />
Private Company<br />
Trust<br />
Other: (Specify)<br />
4 Telephone Number<br />
5 Fax Number<br />
6 Business Physical Address<br />
7 Postal Address: Postal Code:<br />
8 Date Company Established<br />
9 BIT <strong>Registration</strong> Number<br />
10 Company Website Address<br />
11 Tax Clearance Certificate Number:.................................................................................................................................................<br />
Certificate Expiry Date:........................................................................................................................................................................<br />
Tax Certificate Approved Date:.........................................................................................................................................................<br />
6
ROYAL GOVERNMENT OF BHUTAN<br />
MINISTRY OF HEALTH<br />
DEPARTMENT OF MEDICAL SERVICES<br />
DRUGS VACCINE AND EQUIPMENT DIVISION<br />
II.<br />
CONTACT PERSON DETAILS: (Complete for at least two Persons – Preferably Management)<br />
(Compulsory)<br />
CONTACT PERSON 1 CONTACT PERSON 2<br />
1 NAME<br />
2 Job title<br />
4 Telephone number:<br />
5 Fax Number<br />
6 Cellular Number<br />
7 E‐Mail Address<br />
III.<br />
DETAIL OF ALL SHAREHOLDERS AND OWNERS: (Compulsory – APPLICABLE TO OWNERS AND<br />
SHAREHOLDERS ONLY)<br />
Full name CID/passport Citizenship Date of<br />
ownership<br />
% owned<br />
Total 100%<br />
7
ROYAL GOVERNMENT OF BHUTAN<br />
MINISTRY OF HEALTH<br />
DEPARTMENT OF MEDICAL SERVICES<br />
DRUGS VACCINE AND EQUIPMENT DIVISION<br />
IV. REFERENCES (List 5 contracts/projects/organizations, which your Company has been engaged in for the last 2 years) related to medical supplies<br />
Description Location Client Client telephone number Amount<br />
contracted<br />
Completed/expected<br />
completion date<br />
8
ROYAL GOVERNMENT OF BHUTAN<br />
MINISTRY OF HEALTH<br />
DEPARTMENT OF MEDICAL SERVICES<br />
DRUGS VACCINE AND EQUIPMENT DIVISION<br />
V. B ANKING DETAILS (A copy of a cancelled cheque must be attached‐ Compulsory)<br />
Name of Account Holder:<br />
Bank:<br />
Type of Account:<br />
Account Number:<br />
It is hereby confirmed that these details<br />
have been verified<br />
Bank stamp here<br />
NB: It is the <strong>Supplier</strong>’s responsibility to<br />
ensure that the details provided are correct<br />
Bank Official Name: ___________________________________________________<br />
Name of the Bank: _____________________________________________________<br />
Contact Details: ________________________________________________________<br />
9
VI.<br />
ROYAL GOVERNMENT OF BHUTAN<br />
MINISTRY OF HEALTH<br />
DEPARTMENT OF MEDICAL SERVICES<br />
DRUGS VACCINE AND EQUIPMENT DIVISION<br />
DETAILS OF PERSON(S) AUTHORIZED TO ACT ON BEHALF OF THE COMPANY (Mandatory)<br />
RESOLUTION OF OWNERS/DIRECTORS/ MEMBERS/PARTNERS<br />
RESOLUTION of a meeting of the Board of *Directors / Members / Partners/ Owners of:<br />
_____________________________________________________________________________________________________________________<br />
(Legally correct full name and registration number of the Enterprise, if applicable)<br />
Held at ___________________________________________ (Place)<br />
On ________________________________________________ (Date)<br />
RESOLVED that:<br />
1. The Company submits an application to the <strong>DVED</strong> for registration on <strong>DVED</strong>’s <strong>Supplier</strong> Register.<br />
2. *Mr/Mrs/Ms: _______________________________________________________________<br />
in *his/her Capacity as: _________________________________________ (Position in the Enterprise) and who will sign<br />
as follows: (insert specimen signature)_________________be, and is hereby, authorised to sign any documents<br />
and/or correspondence in connection with and relating to the Application <strong>Form</strong> as well as to sign any<br />
Contract, and any and all documentation on behalf of the Company.<br />
Name Capacity Signature<br />
Note:<br />
1. * Delete which is not applicable<br />
2. This resolution must be signed by all the Directors / Members / Partners and Owners of the Bidding Enterprise<br />
3. Should the number of Directors /Members/Partners and Owners exceed the space available above,<br />
additional names and signatures must be supplied on a separate page<br />
Enterprise stamp:<br />
Attach power of attorney:<br />
10
VII.<br />
DECLARATION:<br />
ROYAL GOVERNMENT OF BHUTAN<br />
MINISTRY OF HEALTH<br />
DEPARTMENT OF MEDICAL SERVICES<br />
DRUGS VACCINE AND EQUIPMENT DIVISION<br />
By completing this application form, the Company declares that:<br />
1. All the information provided in this application is true and correct.<br />
2. The Company will, without protest submit itself to procedures instituted by the <strong>DVED</strong><br />
3. The Company will, if requested to do so supply further information and documentary evidence for<br />
scrutiny.<br />
4. The Company will update their registration particulars whenever a significant change in their details<br />
occurs.<br />
5. The Company acknowledges that any false information provided can lead to disqualification from the<br />
<strong>Supplier</strong><br />
6. Register and being listed on <strong>DVED</strong> supplier list.<br />
7. The Company acknowledges that it can be penalized for poor performance.<br />
Is there any relationship between your organization and any of the <strong>DVED</strong> officials/staffs?<br />
Yes<br />
No<br />
If yes, please specify nature of relationship and name of person<br />
Family Friend Business Partner<br />
Full Name: Full Name: Full Name: Full Name:<br />
Duly authorized to sign on behalf of: ___ ________________________ (Name of Company)<br />
The undersigned who warrants that he / she is duly authorized to do so on behalf of the Company, confirms<br />
that the contents of the application are within my personal knowledge and are to the best of my belief both<br />
true and correct.<br />
Signature (Affix a legal<br />
stamp)<br />
Full Name Capacity Date<br />
11