External Fixation System TenXor - Stryker
External Fixation System TenXor - Stryker
External Fixation System TenXor - Stryker
You also want an ePaper? Increase the reach of your titles
YUMPU automatically turns print PDFs into web optimized ePapers that Google loves.
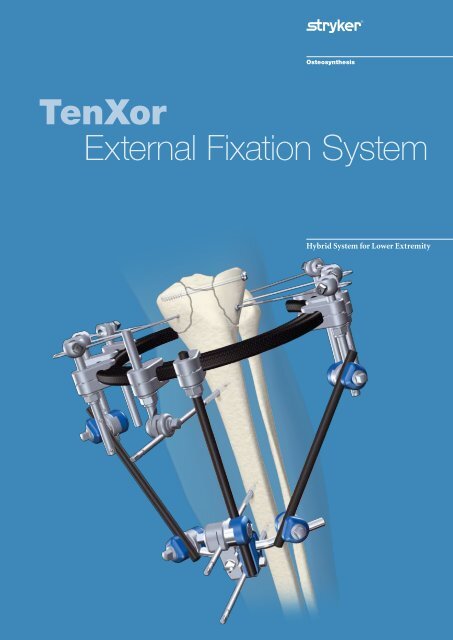
<strong>TenXor</strong><br />
<strong>External</strong> <strong>Fixation</strong> <strong>System</strong><br />
Hybrid <strong>System</strong> for Lower Extremity
Contents<br />
Page<br />
1. Introduction 3<br />
2. Features & Benefits 4<br />
Ease of Use 4<br />
Simple Adaptation 5<br />
Modularity 5<br />
Free Wire Placement 6<br />
Simple Instrumentation 6<br />
3. Relative Indications & Contraindications 7<br />
Relative Indications 7<br />
Relative Contraindications 7<br />
4. Related <strong>Stryker</strong> Products 8<br />
Hoffmann II Compact 8<br />
Asnis III Cannulated Screws 8<br />
AxSOS Locking Plate <strong>System</strong> 8<br />
Ordering Information – Implants 9<br />
Ordering Information – Instruments 10<br />
Additional Information 11<br />
2
Introduction<br />
In severe trauma cases, it<br />
is necessary to have a good<br />
partnership, whether it is a<br />
colleague in the surgical theatre<br />
or an effective and trustworthy<br />
system that allows you to bring the<br />
focus to where it belongs - on the<br />
patient.<br />
The <strong>TenXor</strong> system, a hybrid<br />
<strong>External</strong> Fixator for peri- and<br />
intra-articular fractures of the<br />
lower extremity is especially<br />
designed to fulfill the needs of<br />
today’s surgeons. The advanced<br />
technology helps to provide<br />
simplicity and efficiency of<br />
application.<br />
The right focus - possible<br />
with the <strong>TenXor</strong> system.<br />
Thanks to technical features<br />
like the patented* “Snap Fit”<br />
mechanism, adaptation of height<br />
from the Wire Post and an<br />
unlimited range of motion on<br />
the ring, the <strong>TenXor</strong> delivers the<br />
features surgeons need during a<br />
trauma procedure. Focus is on<br />
the patient and their injuries. The<br />
<strong>TenXor</strong> adapts to the treatment<br />
requirements of the injury<br />
without compromising the clinical<br />
outcome.<br />
During wire insertion,<br />
concentration can be completely<br />
on the fracture and the soft<br />
tissues. The <strong>TenXor</strong> provides the<br />
possibility to adapt the frame even<br />
for non parallel wires.<br />
Do you have a need to change<br />
the frame elasticity during the<br />
treatment?<br />
The compatibility with the<br />
modular Hoffmann II system and<br />
Monotube TRIAX system allows<br />
adaptation of frame elasticity to<br />
the healing process.<br />
The <strong>TenXor</strong> system is a part of<br />
the Hoffmann II family. The<br />
combination of simplicity and<br />
versatility solves your daily<br />
challenges in the surgical theatre<br />
more effectively.<br />
* US Patents 6,080,153; 5,752,954 and<br />
EP 0 700 664<br />
3
Features & Benefits<br />
Ease of Use<br />
The “Snap Fit” mechanism is designed<br />
to provide a quick, easy and secure<br />
frame construction. With a small<br />
number of components and a few<br />
bolts and nuts to tighten, working on<br />
the frame is much facilitated. The<br />
minimal instrumentation needed<br />
is compatible with the Hoffmann II<br />
instrumentation.<br />
High technology carbon material<br />
of the rings provides a better<br />
radiolucency and the etched end of<br />
the olive wires allow a simple removal<br />
without additional x-ray control.<br />
4
Simple Adaptation<br />
The 5/8 open carbon rings facilitate<br />
the selection of the ring size by<br />
sliding it over the extremity directly<br />
during surgery. Due to the “Snap<br />
Fit” mechanism, absolutely no<br />
pre-assembling is necessary. All<br />
components have direct positioning<br />
access to the ring construction.<br />
Additionally the “Snap Fit”<br />
mechanism provides the possibility<br />
to add or remove components to<br />
create the optimal stability during the<br />
treatment with a “Click”.<br />
Modularity<br />
The compatibility with the<br />
Monotube TRIAX and the<br />
Hoffmann II systems provide the<br />
adaptation of elasticity during<br />
treatment. It also adds the option of<br />
independent pin placement and joint<br />
bridging if desired. The “Snap Fit”<br />
mechanism with a “Click” delivers the<br />
solution of a dynamic system.<br />
For more information we refer to the<br />
Hoffmann II brochure (ref.-no. 5075-<br />
1-000) and the Monotube TRIAX<br />
brochure (ref.-no. 5075-2-500).<br />
5
Features & Benefits<br />
Free Wire<br />
Placement<br />
The <strong>TenXor</strong> system has been designed<br />
for effective wire placement adaptable<br />
to fractures and anatomical needs.<br />
The wires can be placed prior and<br />
independently to the frame assembling.<br />
The Ring Clamp provides unlimited<br />
positioning on the ring and the<br />
attachable Wire Post has a height<br />
adjustability up to 2.5 cm with<br />
angulations of +/-20°.<br />
Simple<br />
Instrumentation<br />
Only a few instruments and the<br />
compatibility with the<br />
Hoffmann II instruments facilitate the<br />
work significantly.<br />
The self-explanatory wire tensioner<br />
renders an optimal tensioning of the<br />
wires.<br />
<strong>TenXor</strong><br />
Your system for effective solutions.<br />
6
Relative Indications & Contraindications<br />
Since external fixation devices are<br />
often used in emergency situations to<br />
treat patients with acute injuries, there<br />
are no absolute contraindications for<br />
use. The surgeon’s education, training<br />
and professional judgement must<br />
be relied upon to choose the most<br />
appropriate device and treatment for<br />
each individual patient.<br />
If uncertainly exists with regard to the<br />
anatomic location of the neurovascular<br />
structures due to post-traumatic<br />
destruction, the device should be used<br />
with extreme caution. Under these<br />
circumstances, the wires and pins<br />
should be inserted under direct vision.<br />
Relative<br />
Indications<br />
• Severe multi fragmentary tibia<br />
plateau fractures, e.g. Schatzker<br />
type (4), 5, 6<br />
• Severe multi fragmentary pilon<br />
fractures, e.g. Rüedi & Allgöwer type<br />
2, 3<br />
• Severe distal femur fractures, e.g. AO<br />
33B, 33C<br />
• Secondary fixation after failure or<br />
infection of primary fixation<br />
• Less complex fracture pattern with<br />
severe soft tissue damage, e.g.<br />
Tscherne type C2, C3,<br />
Gustillo Andersen type (2), 3<br />
Relative<br />
Indications<br />
• Patients with compromised immune<br />
system<br />
• Non compliant patients who would<br />
not be able to ensure proper wire and<br />
pin care<br />
• Pre-existing internal fixation<br />
that prohibits proper wire or pin<br />
placement<br />
• Bone pathology precluding pin<br />
fixation<br />
7
Related <strong>Stryker</strong> Products<br />
Hoffmann II Compact<br />
The Hoffmann II Compact <strong>External</strong><br />
<strong>Fixation</strong> system gives the surgeon the<br />
ability to treat a variety of fractures in<br />
the wrist, forearm and foot. This low<br />
profile, modular system is compatible<br />
with the <strong>TenXor</strong> system and adapts<br />
easily to the patients anatomy and soft<br />
tissues.<br />
For more information, contact your<br />
local <strong>Stryker</strong> Representative and<br />
ask for the Hoffmann II Compact<br />
brochure (ref.no. 5075-1-500).<br />
Asnis III Cannulated Screws<br />
This new generation of Cannulated<br />
Screws has been designed to optimize<br />
surgical outcomes while simplifying<br />
procedures.<br />
For more information, contact your<br />
local <strong>Stryker</strong> Representative and ask for<br />
the ASNIS III brochure<br />
(ref.no. 982187).<br />
AxSOS Locking Plate <strong>System</strong><br />
The AxSOS Locking Plate <strong>System</strong> is<br />
designed to treat periarticular or intraarticular<br />
fractures of the Proximal<br />
Humerus, Distal Femur, Proximal<br />
Tibia and the Distal Tibia. The system<br />
design is based on clinical input from<br />
an international panel of experienced<br />
surgeons, data from literature, and<br />
both practical and biomechanical<br />
testing. The anatomical shape, the<br />
fixed screw trajectory, and high surface<br />
quality take into account the current<br />
demands of clinical physicians for<br />
appropriate fixation, high fatigue<br />
strength and minimal soft tissue<br />
damage.<br />
Proximal Lateral<br />
Tibial Plate<br />
For more information contact your<br />
local <strong>Stryker</strong> Representative and ask for<br />
the relevant Operative Technique.<br />
Distal Anterolateral<br />
Tibial Plate<br />
8
Ordering Information – Implants<br />
REF<br />
Description<br />
Ring Components<br />
4936-0-015 Open Ring, 150mm<br />
4936-0-018 Open Ring, 180mm<br />
4936-0-021 Open Ring, 210mm<br />
4936-2-010 Ring Clamp<br />
4936-2-040 Wire Post, 1.5, 2.0mm<br />
4936-2-050 Wire Post, short, 1.5, 2.0mm<br />
4936-2-030 Pin Post, 4,5,6mm<br />
4936-2-920 Ring to Monotube Triax Tube Clamp, blue, 20mm<br />
4936-2-925 Ring to Monotube Triax Tube Clamp, red, 25mm<br />
Wires<br />
5101-1-450 Wire 450mm, 1.5mm<br />
5101-2-450 Wire 450mm, 2.0mm<br />
4936-1-320 Wire etched, with olive, 1.5mm<br />
4936-1-340 Wire etched, with olive, 2.0mm<br />
9
Ordering Information – Instruments<br />
REF<br />
Description<br />
4936-9-010 <strong>TenXor</strong> Wire Tensioner<br />
4936-9-020 Wire Cutting and Bending Plier , max. 2.0mm<br />
4936-9-035 Quick Capture Spanner Wrench, 13mm<br />
4936-9-040 Split Wire Sleeve<br />
4936-9-050 Stabilization Wrench<br />
4936-9-060 T-Wrench, 7mm<br />
4936-9-070 Cardan Wrench, 7mm<br />
4920-9-036 Spanner Wrench, 7mm<br />
4936-9-900 Storage Case - <strong>TenXor</strong><br />
10
Additional Information<br />
HydroSet<br />
Injectable HA<br />
Indications<br />
HydroSet is a self-setting calcium<br />
phosphate cement indicated to fill<br />
bony voids or gaps of the skeletal<br />
system (i.e. extremities, craniofacial,<br />
spine, and pelvis). These defects may<br />
be surgically created or osseous defects<br />
created from traumatic injury to the<br />
bone. HydroSet is indicated only<br />
for bony voids or gaps that are not<br />
intrinsic to the stability of the bony<br />
structure.<br />
HydroSet cured in situ provides an<br />
open void/gap filler than can augment<br />
provisional hardware (e.g K-Wires,<br />
Plates, Screws) to help support<br />
bone fragments during the surgical<br />
procedure. The cured cement acts<br />
only as a temporary support media<br />
and is not intended to provide<br />
structural support during the healing<br />
process.<br />
Tibial Plateau Void Filling<br />
Scanning Electron Microscope Image<br />
of Hydroset material crystalline<br />
microstructure at 15000x<br />
magnification<br />
HydroSet is an injectable,<br />
sculptable and fast-setting bone<br />
substitute. HydroSet is a calcium<br />
phosphate cement that converts to<br />
hydroxyapatite, the principle mineral<br />
component of bone. The crystalline<br />
structure and porosity of HydroSet<br />
makes it an effective osteoconductive<br />
and osteointegrative material, with<br />
excellent biocompatibility and<br />
mechanical properties 1 . HydroSet<br />
was specifically formulated to set in<br />
a wet field environment and exhibits<br />
outstanding wet-field characteristics 2 .<br />
The chemical reaction that occurs as<br />
HydroSet hardens does not release heat<br />
that could be potentially damaging<br />
to the surrounding tissue. Once set,<br />
HydroSet can be drilled and tapped<br />
to augment provisional hardware<br />
placement during the surgical<br />
procedure. After implantation, the<br />
HydroSet is remodeled over time at a<br />
rate that is dependent on the size of the<br />
defect and the average age and general<br />
health of the patient.<br />
Advantages<br />
Injectable or Manual<br />
Implantation<br />
HydroSet can be easily implanted via<br />
simple injection or manual application<br />
techniques for a variety of indications.<br />
Fast Setting<br />
HydroSet has been specifically designed<br />
to set quickly once implanted under<br />
normal physiological conditions,<br />
potentially minimizing procedure<br />
time.<br />
Isothermic<br />
HydroSet does not release any heat as<br />
it sets, preventing potential thermal<br />
injury.<br />
Excellent Wet-Field<br />
Characteristics<br />
HydroSet is chemically formulated<br />
to set in a wet field environment<br />
eliminating the need to meticulously<br />
dry the operative site prior to<br />
implantation. 2<br />
Osteoconductive<br />
The composition of hydroxyapitite<br />
closely matches that of bone mineral<br />
thus imparting osteoconductive<br />
properties. 3<br />
Augmentation of Provisional<br />
Hardware during surgical<br />
procedure<br />
HydroSet can be drilled and tapped<br />
to accommodate the placement of<br />
provisional hardware.<br />
References<br />
1. Chow, L, Takagi, L. A Natural Bone Cement –<br />
A Laboratory Novelty Led to the Development of<br />
Revolutionary New Biomaterials. J. Res. Natl. Stand.<br />
Technolo. 106, 1029-1033 (2001).<br />
2. 1808.E703. Wet field set penetration<br />
(Data on file at <strong>Stryker</strong>)<br />
3. Dickson, K.F., et al. The Use of BoneSource<br />
Hydroxyapatite Cement for Traumatic Metaphyseal<br />
Bone Void Filling. J Trauma 2002; 53:1103-1108.<br />
Note:<br />
Screw fixation must be provided<br />
by bone<br />
11<br />
Ordering Information<br />
Ref Description<br />
397003 3cc HydroSet<br />
397005 5cc HydroSet<br />
397010 10cc HydroSet<br />
397015 15cc HydroSet<br />
Note:<br />
For more detailed information<br />
refer to Litterature No. 90-07900.
<strong>Stryker</strong> Trauma AG<br />
Bohnackerweg 1<br />
CH-2545 Selzach<br />
Switzerland<br />
www.osteosynthesis.styker.com<br />
This document is intended solely for the use of healthcare professionals. A surgeon must always rely on his or her<br />
own professional clinical judgment when deciding whether to use a particular product when treating a particular<br />
patient. <strong>Stryker</strong> does not dispense medical advice and recommends that surgeons be trained in the use of any<br />
particular product before using it in surgery. The information presented in this brochure is intended to<br />
demonstrate a <strong>Stryker</strong> product. Always refer to the package insert, product label and/or user instructions including<br />
the instructions for Cleaning and Sterilization (if applicable) before using any <strong>Stryker</strong> products. Products may not<br />
be available in all markets. Product availability is subject to the regulatory or medical practices that govern<br />
individual markets. Please contact your <strong>Stryker</strong> representative if you have questions about the availability of<br />
<strong>Stryker</strong> products in your area.<br />
<strong>Stryker</strong> Corporation or its divisions or other corporate affiliated entities own, use or have applied for the following<br />
trademarks or service marks: <strong>Stryker</strong>, Asnis, AxSOS, Hoffmann, HydroSet, <strong>TenXor</strong>.<br />
All other trademarks are trademarks of their respective owners or holders.<br />
The products listed above are CE marked.<br />
Literature Number: 5075-3-000<br />
LOT F2209<br />
Copyright © 2009 <strong>Stryker</strong>