Epinephrine and Dexamethasone in Children with Bronchiolitis
Epinephrine and Dexamethasone in Children with Bronchiolitis
Epinephrine and Dexamethasone in Children with Bronchiolitis
Create successful ePaper yourself
Turn your PDF publications into a flip-book with our unique Google optimized e-Paper software.
The new engl<strong>and</strong> journal of medic<strong>in</strong>e<br />
Assessments<br />
The research nurse recorded the patient’s RDAI<br />
score, respiratory rate, heart rate, <strong>and</strong> oxygen saturation<br />
<strong>in</strong> ambient air at basel<strong>in</strong>e, between the<br />
two nebulizations, <strong>and</strong> at 60, 90, 120, 180, <strong>and</strong><br />
240 m<strong>in</strong>utes; rectal temperature at 120 <strong>and</strong> 240<br />
m<strong>in</strong>utes (or at discharge); blood pressure at 240<br />
m<strong>in</strong>utes or at discharge; <strong>and</strong> any side effects<br />
throughout the observation period <strong>in</strong> the emergency<br />
department. Us<strong>in</strong>g a st<strong>and</strong>ardized telephone<br />
follow-up procedure, 18 the research nurse obta<strong>in</strong>ed<br />
data regard<strong>in</strong>g compliance <strong>with</strong> adm<strong>in</strong>istration<br />
of study medication after discharge <strong>and</strong> health<br />
care visits, as well as details about the <strong>in</strong>fant’s feed<strong>in</strong>g,<br />
sleep, breath<strong>in</strong>g, <strong>and</strong> cough<strong>in</strong>g. Follow-up by<br />
telephone was performed daily until day 7, then<br />
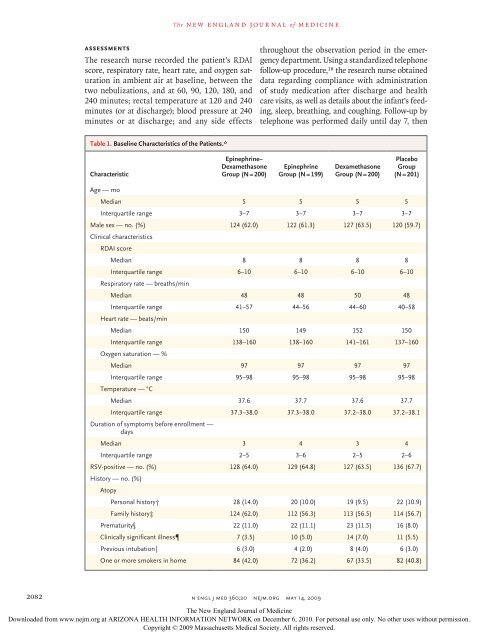
Table 1. Basel<strong>in</strong>e Characteristics of the Patients.*<br />
Characteristic<br />
<strong>Ep<strong>in</strong>ephr<strong>in</strong>e</strong>–<br />
<strong>Dexamethasone</strong><br />
Group (N = 200)<br />
<strong>Ep<strong>in</strong>ephr<strong>in</strong>e</strong><br />
Group (N = 199)<br />
<strong>Dexamethasone</strong><br />
Group (N = 200)<br />
Placebo<br />
Group<br />
(N = 201)<br />
Age — mo<br />
Median 5 5 5 5<br />
Interquartile range 3–7 3–7 3–7 3–7<br />
Male sex — no. (%) 124 (62.0) 122 (61.3) 127 (63.5) 120 (59.7)<br />
Cl<strong>in</strong>ical characteristics<br />
RDAI score<br />
Median 8 8 8 8<br />
Interquartile range 6–10 6–10 6–10 6–10<br />
Respiratory rate — breaths/m<strong>in</strong><br />
Median 48 48 50 48<br />
Interquartile range 41–57 44–56 44–60 40–58<br />
Heart rate — beats/m<strong>in</strong><br />
Median 150 149 152 150<br />
Interquartile range 138–160 138–160 141–161 137–160<br />
Oxygen saturation — %<br />
Median 97 97 97 97<br />
Interquartile range 95–98 95–98 95–98 95–98<br />
Temperature — °C<br />
Median 37.6 37.7 37.6 37.7<br />
Interquartile range 37.3–38.0 37.3–38.0 37.2–38.0 37.2–38.1<br />
Duration of symptoms before enrollment —<br />
days<br />
Median 3 4 3 4<br />
Interquartile range 2–5 3–6 2–5 2–6<br />
RSV-positive — no. (%) 128 (64.0) 129 (64.8) 127 (63.5) 136 (67.7)<br />
History — no. (%)<br />
Atopy<br />
Personal history† 28 (14.0) 20 (10.0) 19 (9.5) 22 (10.9)<br />
Family history‡ 124 (62.0) 112 (56.3) 113 (56.5) 114 (56.7)<br />
Prematurity§ 22 (11.0) 22 (11.1) 23 (11.5) 16 (8.0)<br />
Cl<strong>in</strong>ically significant illness 7 (3.5) 10 (5.0) 14 (7.0) 11 (5.5)<br />
Previous <strong>in</strong>tubation‖ 6 (3.0) 4 (2.0) 8 (4.0) 6 (3.0)<br />
One or more smokers <strong>in</strong> home 84 (42.0) 72 (36.2) 67 (33.5) 82 (40.8)<br />
2082<br />
n engl j med 360;20 nejm.org may 14, 2009<br />
The New Engl<strong>and</strong> Journal of Medic<strong>in</strong>e<br />
Downloaded from www.nejm.org at ARIZONA HEALTH INFORMATION NETWORK on December 6, 2010. For personal use only. No other uses <strong>with</strong>out permission.<br />
Copyright © 2009 Massachusetts Medical Society. All rights reserved.