The 2010 Nobel Prize in Chemistry - Platinum Metals Review
The 2010 Nobel Prize in Chemistry - Platinum Metals Review
The 2010 Nobel Prize in Chemistry - Platinum Metals Review
Create successful ePaper yourself
Turn your PDF publications into a flip-book with our unique Google optimized e-Paper software.
doi:10.1595/147106711X558301<br />
•Plat<strong>in</strong>um <strong>Metals</strong> Rev., 2011, 55, (2)•<br />
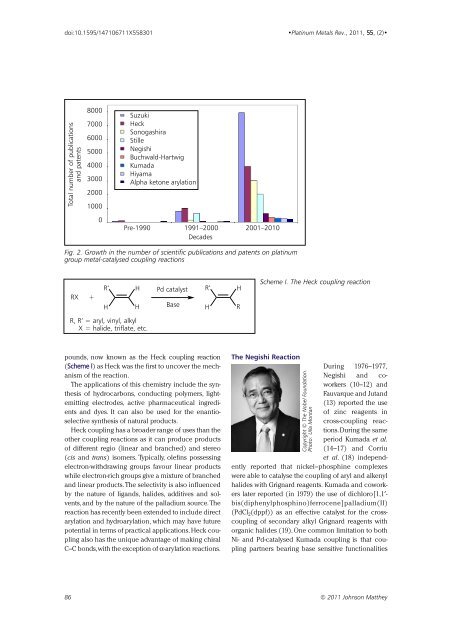
Total number of publications<br />
and patents<br />
8000<br />
7000<br />
6000<br />
5000<br />
4000<br />
3000<br />
2000<br />
1000<br />
0<br />
Suzuki<br />
Heck<br />
Sonogashira<br />
Stille<br />
Negishi<br />
Buchwald-Hartwig<br />
Kumada<br />
Hiyama<br />
Alpha ketone arylation<br />
Pre-1990 1991–2000 2001–<strong>2010</strong><br />
Decades<br />
Fig. 2. Growth <strong>in</strong> the number of scientific publications and patents on plat<strong>in</strong>um<br />
group metal-catalysed coupl<strong>in</strong>g reactions<br />
RX +<br />
R’ H<br />
H<br />
H<br />
Pd catalyst<br />
Base<br />
R’ H<br />
H<br />
R<br />
Scheme I. <strong>The</strong> Heck coupl<strong>in</strong>g reaction<br />
R, R’ = aryl, v<strong>in</strong>yl, alkyl<br />
X = halide, triflate, etc.<br />
pounds, now known as the Heck coupl<strong>in</strong>g reaction<br />
(Scheme I) as Heck was the first to uncover the mechanism<br />
of the reaction.<br />
<strong>The</strong> applications of this chemistry <strong>in</strong>clude the synthesis<br />
of hydrocarbons, conduct<strong>in</strong>g polymers, lightemitt<strong>in</strong>g<br />
electrodes, active pharmaceutical <strong>in</strong>gredients<br />
and dyes. It can also be used for the enantioselective<br />
synthesis of natural products.<br />
Heck coupl<strong>in</strong>g has a broader range of uses than the<br />
other coupl<strong>in</strong>g reactions as it can produce products<br />
of different regio (l<strong>in</strong>ear and branched) and stereo<br />
(cis and trans) isomers. Typically, olef<strong>in</strong>s possess<strong>in</strong>g<br />
electron-withdraw<strong>in</strong>g groups favour l<strong>in</strong>ear products<br />
while electron-rich groups give a mixture of branched<br />
and l<strong>in</strong>ear products.<strong>The</strong> selectivity is also <strong>in</strong>fluenced<br />
by the nature of ligands, halides, additives and solvents,<br />
and by the nature of the palladium source.<strong>The</strong><br />
reaction has recently been extended to <strong>in</strong>clude direct<br />
arylation and hydroarylation, which may have future<br />
potential <strong>in</strong> terms of practical applications. Heck coupl<strong>in</strong>g<br />
also has the unique advantage of mak<strong>in</strong>g chiral<br />
C–C bonds,with the exception of α-arylation reactions.<br />
<strong>The</strong> Negishi Reaction<br />
Copyright © <strong>The</strong> <strong>Nobel</strong> Foundation.<br />
Photo: Ulla Montan<br />
Dur<strong>in</strong>g 1976–1977,<br />
Negishi and coworkers<br />
(10–12) and<br />
Fauvarque and Jutand<br />
(13) reported the use<br />
of z<strong>in</strong>c reagents <strong>in</strong><br />
cross-coupl<strong>in</strong>g reactions.Dur<strong>in</strong>g<br />
the same<br />
period Kumada et al.<br />
(14–17) and Corriu<br />
et al. (18) <strong>in</strong>dependently<br />
reported that nickel–phosph<strong>in</strong>e complexes<br />
were able to catalyse the coupl<strong>in</strong>g of aryl and alkenyl<br />
halides with Grignard reagents. Kumada and coworkers<br />
later reported (<strong>in</strong> 1979) the use of dichloro[1,1′-<br />
bis(diphenylphosph<strong>in</strong>o)ferrocene]palladium(II)<br />
(PdCl 2 (dppf)) as an effective catalyst for the crosscoupl<strong>in</strong>g<br />
of secondary alkyl Grignard reagents with<br />
organic halides (19).One common limitation to both<br />
Ni- and Pd-catalysed Kumada coupl<strong>in</strong>g is that coupl<strong>in</strong>g<br />
partners bear<strong>in</strong>g base sensitive functionalities<br />
86 © 2011 Johnson Matthey