Cardiac Ablation for Type I Atrial Flutter - Boston Scientific
Cardiac Ablation for Type I Atrial Flutter - Boston Scientific
Cardiac Ablation for Type I Atrial Flutter - Boston Scientific
Create successful ePaper yourself
Turn your PDF publications into a flip-book with our unique Google optimized e-Paper software.
<strong>Cardiac</strong> <strong>Ablation</strong> <strong>for</strong> <strong>Type</strong> I <strong>Atrial</strong> <strong>Flutter</strong><br />
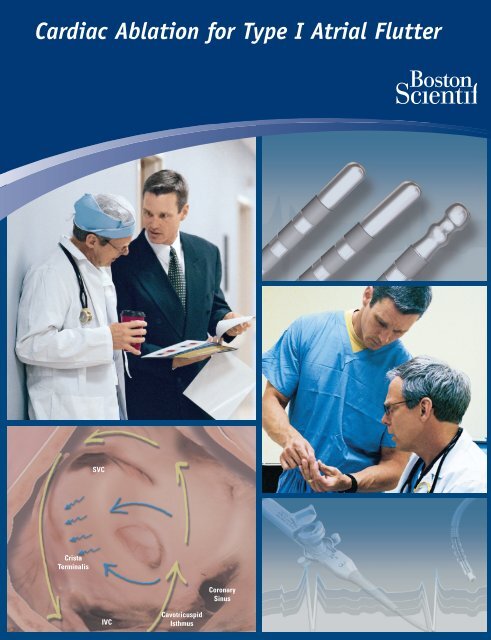
SVC<br />
Crista<br />
Terminalis<br />
Coronary<br />
Sinus<br />
IVC<br />
Cavotricuspid<br />
Isthmus
BOSTON SCIENTIFIC: Blazer II XP <strong>Cardiac</strong> <strong>Ablation</strong> Catheter; EPT-1000XP Controller<br />
SUMMARY OF SAFETY AND EFFECTIVENESS DATA<br />
PMA NUMBER: P020025 DATE OF APPROVAL: 25-Aug-2003<br />
RESULTS* Percentage # of Procedures<br />
Acute Success 94% 250<br />
Six Month Success 96% 151<br />
Major Complications* 8% 250<br />
Pericardial effusion/tamponade 0<br />
Intra-cardiac thrombus 0<br />
TIA 1<br />
CVA 2<br />
Mean ± SD<br />
# of Procedures<br />
Total Saline infused 0 ml 250<br />
Total # of RF Applications/Procedure 11.5 ± 10.6 209<br />
Total Procedure Time 2.1 ± 1.3 hours 234<br />
Total Fluoroscopy Time 28.5 ± 20.2 minutes 232<br />
SUMMARY OF CLINICAL STUDIES*<br />
OBJECTIVE<br />
STUDY DESIGN<br />
The objective of the study was to evaluate the safety and efficacy of the Blazer II XP <strong>Cardiac</strong><br />
<strong>Ablation</strong> Catheter and EPT-1000XP <strong>Cardiac</strong> <strong>Ablation</strong> Controller and Accessories <strong>for</strong> radiofrequency<br />
ablation of sustained or recurrent type I atrial flutter.<br />
The study was a prospective, multi-center, single-arm study using objective per<strong>for</strong>mance criteria and<br />
historical control data from the medical literature. Clinical efficacy and safety assessments were per<strong>for</strong>med<br />
at one, three and six months, and at one and two years following the index procedure.<br />
STUDY ENDPOINTS<br />
The primary endpoints <strong>for</strong> the study were as follows:<br />
Acute Procedural Success: defined as complete bi-directional isthmus block with noninducible<br />
type I atrial flutter with only the use of the Blazer II XP <strong>Cardiac</strong> <strong>Ablation</strong> Catheter and EPT-1000XP<br />
<strong>Cardiac</strong> <strong>Ablation</strong> Controller and Accessories as assessed at the end of the ablation procedure.<br />
Chronic Effectiveness Success: defined as demonstration of Acute Success and continued<br />
absence of targeted type I atrial flutter <strong>for</strong> the first six months after the ablation procedure.<br />
Procedural Safety: defined by the absence of serious complications associated with the use of<br />
the investigational device within seven days of the ablation procedure.<br />
PATIENT<br />
ACCOUNTABILITY<br />
Patients enrolled in the study 250<br />
Patients not ablated 0<br />
Patients ablated with EPT-1000 XP <strong>Cardiac</strong> <strong>Ablation</strong> System 250<br />
Patients ablated only with EPT-1000 XP <strong>Cardiac</strong> <strong>Ablation</strong> System 247<br />
Patients ablated with EPT-1000 XP <strong>Cardiac</strong> <strong>Ablation</strong> System<br />
and non-investigational catheter* 5<br />
Patients ablated only with non-investigational catheter 2<br />
*Patients were first ablated with the EPT-1000XP <strong>Cardiac</strong> <strong>Ablation</strong> System only. If flutter procedure<br />
could not be completed, then physicians used another catheter to complete the procedure.<br />
These patients were considered acute failures.<br />
STUDY<br />
DEMOGRAPHICS<br />
Male: 205/247 (83%) The average age of the male patients is 60.5 ± 11.1 years.<br />
Female: 42/247 (17%) The average age of the female patients is 63.4 ± 12.4 years.<br />
*For additional in<strong>for</strong>mation, refer to SSE PMA: P020025 The SSE can be found at: http://www.fda.gov/ohrms/dockets<br />
Enter the product name into the “Search” field.<br />
Note: Data reflected is not from a head-to-head clinical study.
BIOSENSE WEBSTER: NaviStar/Celsius ThermoCool® Diagnostic/<strong>Ablation</strong> Deflectable Tip Catheters<br />
SUMMARY OF SAFETY AND EFFECTIVENESS DATA<br />
PMA NUMBER: P030031 DATE OF APPROVAL: 24-Nov-2004<br />
RESULTS* Percentage # of Procedures<br />
Acute Success 85% 186<br />
Six Month Success 93% 147<br />
Major Complications* 15.8% 190<br />
Pericardial effusion/tamponade 4<br />
Intra-cardiac thrombus 2<br />
TIA 1<br />
CVA 0<br />
Mean ± SD<br />
# of Procedures<br />
Total Saline infused 999.7 ± 605.5 ml 169<br />
Total # of RF Applications/Procedure 19 ± 16 188<br />
Total Procedure Time 5.7 ± 2.8 hours 190<br />
Total Fluoroscopy Time 50.2 ± 32.4 minutes 189<br />
SUMMARY OF CLINICAL STUDIES*<br />
OBJECTIVE<br />
STUDY DESIGN<br />
STUDY ENDPOINTS<br />
SUBJECT<br />
ACCOUNTABILITY<br />
SUBJECT<br />
DEMOGRAPHICS<br />
The objective of the study was to determine if the NaviStarT ThermoCool catheter, when used in<br />
conjunction with Carto EP/XP Navigation System, Stockert 70 RF Generator and related accessories,<br />
is safe and effective <strong>for</strong> the treatment of <strong>Type</strong> I atrial flutter in patients age 18 or older.<br />
The study was a prospective, non-randomized, unblinded, multi-center study conducted at 22<br />
investigational sites (21 sites in US; 1 in Canada).<br />
The endpoints <strong>for</strong> the study were as follows:<br />
Procedural safety - defined by the absence of serious complication associated with the use of<br />
the investigational device within seven days of the ablation procedure; and Acute procedural<br />
success - defined as complete bi-directional conduction block (BDB) across the isthmus, and<br />
the inability to induce <strong>Type</strong> I atrial flutter post-procedure.<br />
Long-term freedom from atrial flutter recurrence was not specifically identified as a study endpoint.<br />
Instead, acute procedural success was used as a surrogate endpoint <strong>for</strong> this parameter.<br />
Long-term (defined as 6 months post-treatment) freedom from atrial flutter recurrence in<strong>for</strong>mation<br />
was also collected, in order to enable FDA to assess whether the surrogate endpoint<br />
was reasonable.<br />
Subjects enrolled in study 198<br />
Subjects not ablated with the NaviStar ThermoCool catheter 9<br />
Subjects ablated with NaviStar ThermoCool catheter 190<br />
Subjects ablated with NaviStar ThermoCool catheter<br />
and non-investigational catheter* 19<br />
Subjects ablated only with NaviStar ThermoCool catheter 171<br />
*This category includes enrolled subjects who received ablation therapy with the investigational<br />
catheter at the start of the procedure and <strong>for</strong> whom the investigator then switched to<br />
a non-protocol catheter to complete the procedure. Further, subjects who could not receive<br />
ablation due to investigational device failure are included in this category. These subjects<br />
were considered acute effectiveness failures.<br />
Female: 44/190 (23.2%)<br />
Male: 146/190 (76.8%)<br />
Age Range (years): 18-90 (Mean ± standard deviation 59.8 ± 12.6)<br />
*For additional in<strong>for</strong>mation, refer to SSE PMA: P030031. The SSE can be found at: http://www.fda.gov/ohrms/dockets<br />
Enter the product name into the “Search” field.<br />
Note: Data reflected is not from a head-to-head clinical study.
BIOSENSE WEBSTER: NaviStar DS Catheter and Celsius DS Diagnostic/<strong>Ablation</strong> Deflectable Tip Catheters<br />
SUMMARY OF SAFETY AND EFFECTIVENESS DATA<br />
PMA NUMBER: P010068 DATE OF APPROVAL: 27-Sep-2002<br />
RESULTS* Percentage # of Procedures<br />
Acute Success 90% 182<br />
Six Month Success 94% 112<br />
Major Complications* 7% 182<br />
Pericardial effusion/tamponade<br />
not documented<br />
Intra-cardiac thrombus<br />
not documented<br />
Neurological 2<br />
Mean ± SD<br />
# of Procedures<br />
Total Saline infused 0 n/a<br />
Total # of RF Applications/Procedure 24.1 ± 19.7 182<br />
Total Procedure Time 2.7 ± 2.4 hours 178<br />
Total Fluoroscopy Time 31 ± 30 minutes 176<br />
SUMMARY OF CLINICAL STUDIES*<br />
OBJECTIVE<br />
STUDY DESIGN<br />
STUDY ENDPOINTS<br />
SUBJECT<br />
ACCOUNTABILITY<br />
The objective of the study was to determine if the NaviStar DS catheter, when used in conjunction<br />
with the Stockert 70 RF Generator and related accessories, is safe and effective <strong>for</strong> the treatment<br />
of <strong>Type</strong> I atrial flutter in patients age 18 or older.<br />
The study was a prospective, non-randomized, unblinded, multi-center study conducted at 16<br />
investigational sites.<br />
The endpoints <strong>for</strong> the study were as follows:<br />
Procedural safety - defined by the absence of serious complication associated with the use of<br />
the investigational device within seven days of the ablation procedure; and Acute procedural<br />
success - defined as complete bi-directional conduction block (BDB) across the isthmus, and<br />
the inability to induce typical atrial flutter post-procedure.<br />
Long-term freedom from atrial flutter recurrence was not specifically identified as a study endpoint.<br />
Instead, FDA allowed acute procedural success to be used as a surrogate endpoint <strong>for</strong><br />
this parameter. Long-term (defined as 6 months post-treatment) freedom from atrial flutter<br />
recurrence in<strong>for</strong>mation was also collected, in order to enable FDA to assess whether the surrogate<br />
endpoint was reasonable.<br />
Subjects enrolled in study 191<br />
Subjects not ablated 9<br />
Subjects ablated with NaviStar DS catheter 182<br />
Subjects ablated with NaviStar DS catheter<br />
and non-investigational catheter* 15<br />
Patients found to have atypical atrial flutter post ablation** 1<br />
*Patients were first ablated with the NaviStar only. If flutter procedure could not be completed,<br />
then physicians used another catheter to complete procedure. These patients were<br />
considered acute effectiveness failures.<br />
**This patient was considered an acute effectiveness failure.<br />
SUBJECT<br />
DEMOGRAPHICS<br />
Female: 39/182 (21.43%)<br />
Male: 143/182 (78.57%)<br />
Age Range (years): 37-89 (Mean ± standard deviation 64.27 ± 10.20)<br />
*For additional in<strong>for</strong>mation, refer to SSE PMA: P010068. The SSE can be found at: http://www.fda.gov/ohrms/dockets<br />
Enter the product name into the “Search” field. Note: The EZ Steer Catheter was released under this PMA; substantially<br />
equivalent with a new handle and puller wires. Note: Data reflected is not from a head-to-head clinical study.
Caution: Federal law (USA) restricts these devices to sale by or on the order of a physician. See the appropriate technical manuals<br />
<strong>for</strong> detailed in<strong>for</strong>mation regarding instructions <strong>for</strong> use, indications, contraindications, warnings and precautions, and potential<br />
adverse events. ©2007 <strong>Boston</strong> <strong>Scientific</strong> Corporation or its affiliates. All rights reserved. EPT-10559_04/07<br />
<strong>Boston</strong> <strong>Scientific</strong> Corporation<br />
2710 Orchard Parkway<br />
San Jose, CA 95134<br />
www.bostonscientific.com/electrophysiology<br />
Customer Service<br />
1-888-272-1001