Day 008- Single Displacement and Metal Activity Series Lab
Day 008- Single Displacement and Metal Activity Series Lab
Day 008- Single Displacement and Metal Activity Series Lab
You also want an ePaper? Increase the reach of your titles
YUMPU automatically turns print PDFs into web optimized ePapers that Google loves.
<strong>Day</strong> <strong>008</strong> <strong>Single</strong> <strong>Displacement</strong> <strong>and</strong> <strong>Metal</strong> <strong>Activity</strong> <strong>Series</strong> <strong>Lab</strong><br />
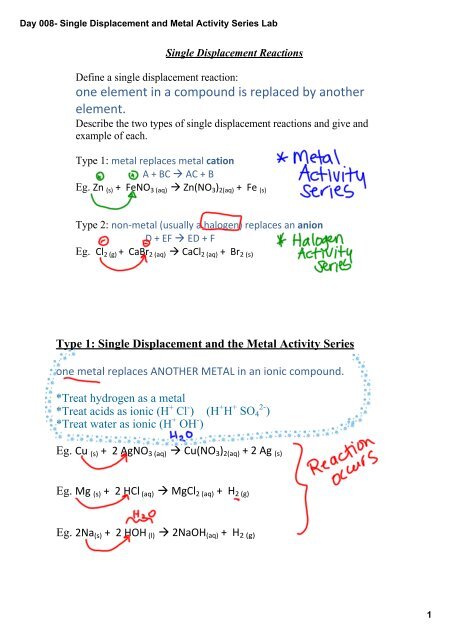
<strong>Single</strong> <strong>Displacement</strong> Reactions<br />
Define a single displacement reaction:<br />
one element in a compound is replaced by another<br />
element.<br />
Describe the two types of single displacement reactions <strong>and</strong> give <strong>and</strong><br />
example of each.<br />
Type 1: metal replaces metal cation<br />
A + BC à AC + B<br />
Eg. Zn (s) + FeNO 3 (aq) à Zn(NO 3 ) 2(aq) + Fe (s)<br />
Type 2: non‐metal (usually a halogen) replaces an anion<br />
D + EF à ED + F<br />
Eg. Cl 2 (g) + CaBr 2 (aq) à CaCl 2 (aq) + Br 2 (s)<br />
Type 1: <strong>Single</strong> <strong>Displacement</strong> <strong>and</strong> the <strong>Metal</strong> <strong>Activity</strong> <strong>Series</strong><br />
one metal replaces ANOTHER METAL in an ionic compound.<br />
*Treat hydrogen as a metal<br />
*Treat acids as ionic (H + Cl ) (H + H + SO 4 2 )<br />
*Treat water as ionic (H + OH )<br />
Eg. Cu (s) + 2 AgNO 3 (aq) à Cu(NO 3 ) 2(aq) + 2 Ag (s)<br />
Eg. Mg (s) + 2 HCl (aq) à MgCl 2 (aq) + H 2 (g)<br />
Eg. 2Na (s) + 2 HOH (l) à 2NaOH (aq) + H 2 (g)<br />
1
<strong>Day</strong> <strong>008</strong> <strong>Single</strong> <strong>Displacement</strong> <strong>and</strong> <strong>Metal</strong> <strong>Activity</strong> <strong>Series</strong> <strong>Lab</strong><br />
<strong>Lab</strong> Investigation Part 1:<br />
Creating An <strong>Activity</strong> <strong>Series</strong> of <strong>Metal</strong>s<br />
Using your observation chart from the lab, order the metals from most to least reactive.<br />
Most _____ _____ _____ _____ _____ Least<br />
2
<strong>Day</strong> <strong>008</strong> <strong>Single</strong> <strong>Displacement</strong> <strong>and</strong> <strong>Metal</strong> <strong>Activity</strong> <strong>Series</strong> <strong>Lab</strong><br />
<strong>Activity</strong> <strong>Series</strong> of <strong>Metal</strong>s See p.140<br />
Although most metals lose electrons in a chemical<br />
reaction they do not do it with the same speed <strong>and</strong><br />
vigor. <strong>Metal</strong>s react differently with different substances.<br />
The more easily a metal atom can lose its electron, the<br />
greater is its reactivity, <strong>and</strong> the more easily oxidized it<br />
is.<br />
The activity series of metals organizes metals from the<br />
most reactive to the least reactive.<br />
The higher the metal in the series, the more reactive it is i.e., its reaction<br />
is fast <strong>and</strong> more exothermic. Remember, exothermic reactions are those<br />
that produce energy like heat <strong>and</strong> light. <strong>Metal</strong>s like gold <strong>and</strong> platinum are<br />
unreactive even in very strong acids.<br />
We can use the activity series to predict the products of single<br />
displacement reactions. In general, an element that is higher in the<br />
activity series will displace an element that is lower.<br />
Hydrogen is included in the series because, like metals, it can be<br />
oxidized to form positive ions.<br />
<strong>Lab</strong> Investigation Part 2:<br />
Using An <strong>Activity</strong> <strong>Series</strong> of <strong>Metal</strong>s<br />
Use your <strong>Metal</strong> <strong>Activity</strong> <strong>Series</strong> to predict the products of the reactions. If no reaction occurs write NR.<br />
3
<strong>Day</strong> <strong>008</strong> <strong>Single</strong> <strong>Displacement</strong> <strong>and</strong> <strong>Metal</strong> <strong>Activity</strong> <strong>Series</strong> <strong>Lab</strong><br />
Using your observation chart from the lab, order the metals from most to least reactive.<br />
Most _____ _____ _____ _____ _____ Least<br />
Use your <strong>Metal</strong> <strong>Activity</strong> <strong>Series</strong> to predict the products for your reactions.<br />
Most _____ _____ _____ _____ _____ Least<br />
How do your lab results compare to the predictions made using the metal activity series?<br />
Type 2: <strong>Single</strong> <strong>Displacement</strong> <strong>and</strong> the Halogen <strong>Activity</strong> <strong>Series</strong><br />
Read the section in your text about <strong>Single</strong> <strong>Displacement</strong><br />
What is the activity series for halogens?<br />
Describe how to use it <strong>and</strong> give two examples.<br />
<strong>Activity</strong> series for Halogens is in the same order as the Periodic<br />
Table<br />
So F > Cl > Br > I<br />
Eg 1:<br />
F 2 (g) + 2NaCl (aq) à 2NaF (aq) + Cl 2 (g)<br />
Eg 2: I 2 (g) + CaBr 2 (aq) à No Rxn<br />
Homework:<br />
<strong>Single</strong> <strong>Displacement</strong>: p. 127 # 21 p. 131 # 22, 23, 24<br />
4