Legacy System 1-2-3 prosthetic catalog February 2011_Project1.qxd
Legacy System 1-2-3 prosthetic catalog February 2011_Project1.qxd
Legacy System 1-2-3 prosthetic catalog February 2011_Project1.qxd
You also want an ePaper? Increase the reach of your titles
YUMPU automatically turns print PDFs into web optimized ePapers that Google loves.
<strong>Legacy</strong> Labeling: Definitions and Descriptions<br />
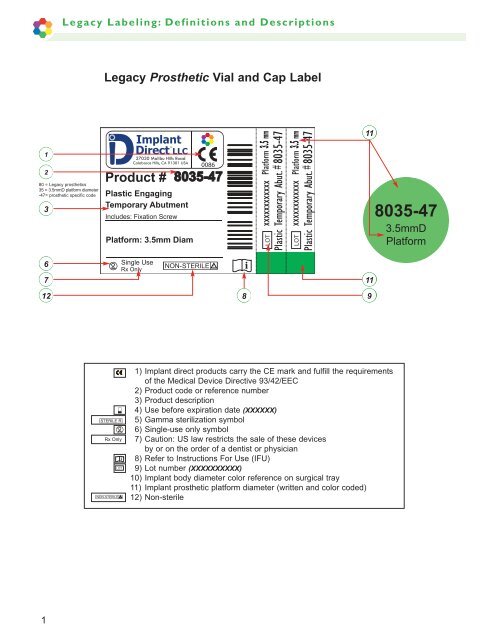
<strong>Legacy</strong> Prosthetic Vial and Cap Label<br />
11<br />
1<br />
2<br />
80 = <strong>Legacy</strong> <strong>prosthetic</strong>s<br />
35 = 3.5mmD platform diameter<br />
-47= <strong>prosthetic</strong> specific code<br />
3<br />
6<br />
7<br />
11<br />
12 8<br />
9<br />
1) Implant direct products carry the CE mark and fulfill the requirements<br />
of the Medical Device Directive 93/42/EEC<br />
2) Product code or reference number<br />
3) Product description<br />
4) Use before expiration date (XXXXXX)<br />
5) Gamma sterilization symbol<br />
6) Single-use only symbol<br />
7) Caution: US law restricts the sale of these devices<br />
by or on the order of a dentist or physician<br />
8) Refer to Instructions For Use (IFU)<br />
9) Lot number (XXXXXXXXXX)<br />
10) Implant body diameter color reference on surgical tray<br />
11) Implant <strong>prosthetic</strong> platform diameter (written and color coded)<br />
12) Non-sterile<br />
1