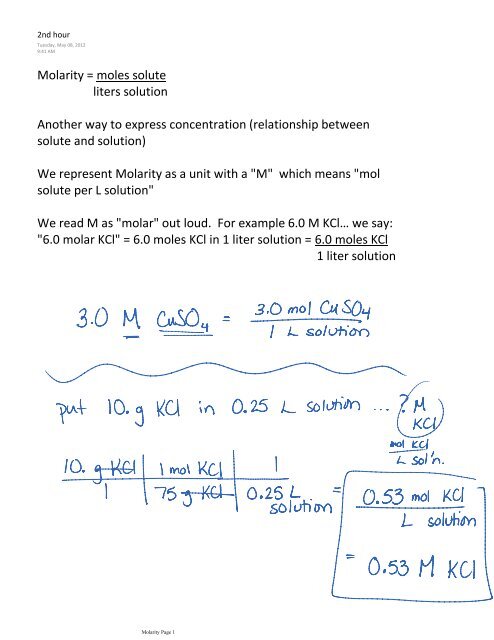
liters solution Molarity = moles solute Another way to express ...
liters solution Molarity = moles solute Another way to express ...
liters solution Molarity = moles solute Another way to express ...
Create successful ePaper yourself
Turn your PDF publications into a flip-book with our unique Google optimized e-Paper software.
2nd hour<br />
Tuesday, May 08, 2012<br />
9:41 AM<br />
<strong>Molarity</strong> = <strong>moles</strong> <strong>solute</strong><br />
<strong>liters</strong> <strong>solution</strong><br />
<strong>Another</strong> <strong>way</strong> <strong>to</strong> <strong>express</strong> concentration (relationship between<br />
<strong>solute</strong> and <strong>solution</strong>)<br />
We represent <strong>Molarity</strong> as a unit with a "M" which means "mol<br />
<strong>solute</strong> per L <strong>solution</strong>"<br />
We read M as "molar" out loud. For example 6.0 M KCl… we say:<br />
"6.0 molar KCl" = 6.0 <strong>moles</strong> KCl in 1 liter <strong>solution</strong> = 6.0 <strong>moles</strong> KCl<br />
1 liter <strong>solution</strong><br />
<strong>Molarity</strong> Page 1
<strong>Molarity</strong> Page 2
7th hour<br />
Tuesday, May 08, 2012<br />
1:45 PM<br />
<strong>Molarity</strong> = <strong>moles</strong> <strong>solute</strong><br />
<strong>liters</strong> <strong>solution</strong><br />
<strong>Another</strong> <strong>way</strong> <strong>to</strong> <strong>express</strong> concentration (relationship between <strong>solute</strong><br />
and <strong>solution</strong>)<br />
We represent <strong>Molarity</strong> as a unit with a "M" which means "mole<br />
<strong>solute</strong> per L <strong>solution</strong>"<br />
We read M as "molar" out loud. For example 6.0 M KCl… we say:<br />
"6.0 molar KCl" = 6.0 <strong>moles</strong> KCl in 1 liter <strong>solution</strong> = 6.0 <strong>moles</strong> KCl<br />
1 liter <strong>solution</strong><br />
<strong>Molarity</strong> Page 3
8th hour<br />
Tuesday, May 08, 2012<br />
1:45 PM<br />
<strong>Molarity</strong> = <strong>moles</strong> <strong>solute</strong><br />
<strong>liters</strong> <strong>solution</strong><br />
<strong>Another</strong> <strong>way</strong> <strong>to</strong> <strong>express</strong> concentration (relationship between <strong>solute</strong><br />
and <strong>solution</strong>)<br />
We represent <strong>Molarity</strong> as a unit with a "M" which means "mol<br />
<strong>solute</strong> per L <strong>solution</strong>"<br />
We read M as "molar" out loud. For example 6.0 M KCl… we say:<br />
"6.0 molar KCl" = 6.0 <strong>moles</strong> KCl in 1 liter <strong>solution</strong> = 6.0 <strong>moles</strong> KCl<br />
1 liter <strong>solution</strong><br />
<strong>Molarity</strong> Page 4
<strong>Molarity</strong> Page 5