Electrode/Electrolyte Interface Studies in Lithium Batteries Using NMR
Electrode/Electrolyte Interface Studies in Lithium Batteries Using NMR
Electrode/Electrolyte Interface Studies in Lithium Batteries Using NMR
You also want an ePaper? Increase the reach of your titles
YUMPU automatically turns print PDFs into web optimized ePapers that Google loves.
Dupré, Cuis<strong>in</strong>ier, and Guyomard<br />
(cont<strong>in</strong>ued from previous page)<br />
the cathode while no correlation was<br />
found for LiF vs. cycle number for the<br />
anode.<br />
In addition to this wider detection<br />
range and <strong>in</strong> order to <strong>in</strong>vestigate<br />
the organic components of the SEI,<br />
13 C <strong>NMR</strong> provides a way to follow the<br />
decomposition reaction of organic<br />
carbonates yield<strong>in</strong>g the organic part of<br />
the SEI as performed on the carbonaceous<br />
anodic electrode <strong>in</strong> C/LiCoO 2 batteries. 34<br />
<strong>NMR</strong> signals assigned to lithium-based<br />
carbon derivatives showed that the<br />
Li-salt reacts with solvent, lead<strong>in</strong>g to<br />
accumulation of decomposition products<br />
on the electrode particles surface. 35<br />
Furthermore, the use of 13 C enriched<br />
ethylene-carbonate and diethylcarbonate<br />
solvents allowed follow<strong>in</strong>g<br />
the decomposition pathway, <strong>in</strong>dicat<strong>in</strong>g<br />
the presence of carbonyl groups <strong>in</strong> SEI<br />
layer, demonstrat<strong>in</strong>g a nucleophilic<br />
attack mechanism on the carbonyl<br />
carbon by one or more radical, alkoxy,<br />
carbanion, or fluor<strong>in</strong>e-conta<strong>in</strong><strong>in</strong>g ionic<br />
species formed from solvent and/or salt<br />
decomposition. These results suggest<br />
a new family of electrolyte breakdown<br />
products, predom<strong>in</strong>antly consist<strong>in</strong>g of<br />
b<strong>in</strong>ary, ternary, and/or quaternary ethertype<br />
compounds (i.e., orthocarbonates<br />
and orthoesters), as well as fluor<strong>in</strong>econta<strong>in</strong><strong>in</strong>g<br />
alkoxy compounds. 34<br />
Most of these studies, done on<br />
diamagnetic materials, rely only on<br />
the <strong>in</strong>terpretation of the chemical shift<br />
to determ<strong>in</strong>e the chemical nature of<br />
the species formed from electrolyte<br />
decomposition, and on the monitor<strong>in</strong>g<br />
of the <strong>in</strong>tegrated <strong>in</strong>tensity to follow<br />
the progress of that reaction. In the<br />
case of transition metal compounds,<br />
<strong>NMR</strong> detection is more complicated,<br />
especially due to fast relaxation time<br />
lead<strong>in</strong>g to broaden<strong>in</strong>g of the signal<br />
caused by the presence of paramagnetic<br />
centers. However the result<strong>in</strong>g <strong>NMR</strong><br />
spectra conta<strong>in</strong> valuable additional<br />
<strong>in</strong>formation on the <strong>in</strong>teraction between<br />
surface species and active electrode<br />
material bulk.<br />
Use of <strong>NMR</strong> to Characterize<br />
<strong>Interface</strong> Species<br />
on Paramagnetic Materials<br />
Magic angle sp<strong>in</strong>n<strong>in</strong>g (MAS) <strong>NMR</strong><br />
is generally used to characterize bulk<br />
materials. The Fermi contact <strong>in</strong>teraction<br />
along with the electron-nucleus dipolar<br />
<strong>in</strong>teractions and their respective<br />
effects on the 6,7 Li <strong>NMR</strong> spectra have<br />
been widely discussed <strong>in</strong> the case of<br />
lithium ions present with<strong>in</strong> the host<br />
matrix of the <strong>in</strong>sertion material. 22,36<br />
The correspond<strong>in</strong>g mechanisms have<br />
also recently been <strong>in</strong>vestigated us<strong>in</strong>g<br />
theoretical calculations. 37-39 Like the<br />
Chemical Shift Anisotropy (CSA), the<br />
electron-nucleus dipolar <strong>in</strong>teraction<br />
scales with the field. At high field, even<br />
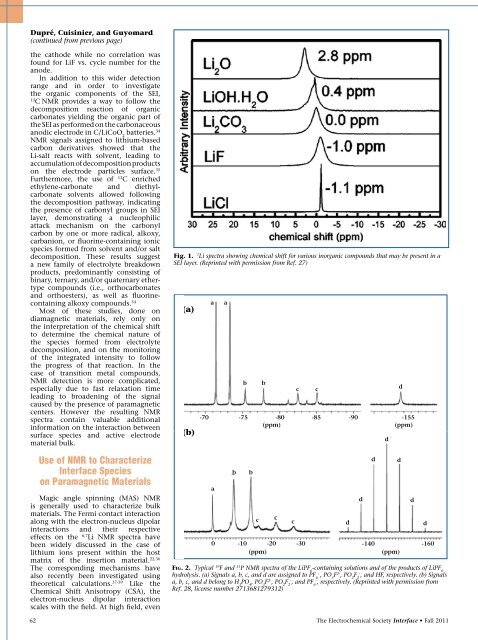
Fig. 1. 7 Li spectra show<strong>in</strong>g chemical shift for various <strong>in</strong>organic compounds that may be present <strong>in</strong> a<br />
SEI layer. (Repr<strong>in</strong>ted with permission from Ref. 27)<br />
(a)<br />
(b)<br />
-70<br />
-70<br />
a<br />
a<br />
a<br />
a<br />
a<br />
a<br />
b b<br />
c c<br />
-75 -80 -85 -90 -155<br />
(ppm)<br />
(ppm)<br />
-75 -80 -85 -90 -155<br />
(ppm)<br />
(ppm)<br />
b b<br />
b b<br />
b b<br />
c c<br />
c<br />
c<br />
c d<br />
d<br />
d<br />
d<br />
0 -10<br />
c<br />
c<br />
-20<br />
c<br />
-30<br />
d<br />
-140<br />
d<br />
-160<br />
(ppm) (ppm)<br />
0 -10 -20 -30<br />
-140 -160<br />
Fig. 2. Typical (ppm) (ppm)<br />
19F and 31P <strong>NMR</strong> spectra of the LiPF -conta<strong>in</strong><strong>in</strong>g solutions and of the products of LiPF 6 6<br />
- hydrolysis. (a) Signals a, b, c, and d are assigned to PF , PO3F 6<br />
2- - , PO F , and HF, respectively. (b) Signals<br />
2 2<br />
a, b, c, and d belong to H PO , PO F 3 4 3 2- - - , PO F , and PF6 , respectively. (Repr<strong>in</strong>ted with permission from<br />
2 2<br />
Ref. 28, license number 2713681279312)<br />
62 The Electrochemical Society <strong>Interface</strong> • Fall 2011<br />
d<br />
d<br />
d<br />
d<br />
d<br />
d<br />
d<br />
d<br />
d<br />
d