TCAs versus placebo - National Center for Biotechnology Information
TCAs versus placebo - National Center for Biotechnology Information
TCAs versus placebo - National Center for Biotechnology Information
You also want an ePaper? Increase the reach of your titles
YUMPU automatically turns print PDFs into web optimized ePapers that Google loves.
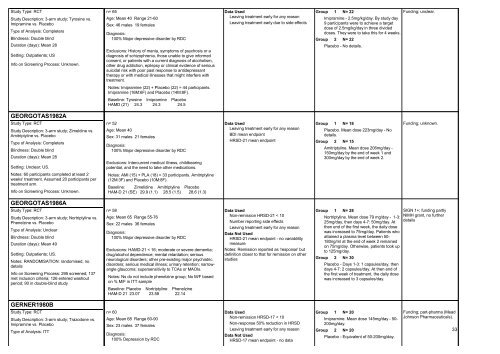
Study Type: RCT<br />
Study Description: 3-arm study; Tyrosine vs.<br />
Imipramine vs. Placebo<br />
Type of Analysis: Completers<br />
Blindness: Double blind<br />
Duration (days): Mean 28<br />
Setting: Outpatients; US<br />
Info on Screening Process: Unknown.<br />
GEORGOTAS1982A<br />
Study Type: RCT<br />
Study Description: 3-arm study; Zimeldine vs.<br />
Amitriptyline vs. Placebo<br />
Type of Analysis: Completers<br />
Blindness: Double blind<br />
Duration (days): Mean 28<br />
Setting: Unclear; US.<br />
Notes: 60 participants completed at least 2<br />
weeks' treatment. Assumed 20 participants per<br />
treatment arm.<br />
Info on Screening Process: Unknown.<br />
GEORGOTAS1986A<br />
Study Type: RCT<br />
Study Description: 3-arm study; Nortriptyline vs.<br />
Phenelzine vs. Placebo<br />
Type of Analysis: Unclear<br />
Blindness: Double blind<br />
Duration (days): Mean 49<br />
Setting: Outpatients; US.<br />
Notes: RANDOMISATION: randomised, no<br />
details<br />
Info on Screening Process: 295 screened; 137<br />
met inclusion criteria; 126 entered washout<br />
period; 90 in double-blind study<br />
GERNER1980B<br />
Study Type: RCT<br />
Study Description: 3-arm study; Trazodone vs.<br />
Imipramine vs. Placebo<br />
Type of Analysis: ITT<br />
Blindness: Double blind<br />
n= 65<br />
Age: Mean 40 Range 21-60<br />
Sex: 46 males 19 females<br />
Diagnosis:<br />
100% Major depressive disorder by RDC<br />
Exclusions: History of mania, symptoms of psychosis or a<br />
diagnosis of schizophrenia, those unable to give in<strong>for</strong>med<br />
consent, or patients with a current diagnosis of alcoholism,<br />
other drug addiction, epilepsy or clinical evidence of serious<br />
suicidal risk with poor past response to antidepressant<br />
therapy or with medical illnesses that might interfere with<br />
treatment.<br />
Notes: Imipramine (22) + Placebo (22) = 44 participants.<br />
Imipramine (16M:6F) and Placebo (14M:8F).<br />
Baseline: Tyrosine Imipramine Placebo<br />
HAMD (21) 24.3 24.3 24.5<br />
n= 52<br />
Age: Mean 40<br />
Sex: 31 males 21 females<br />
Diagnosis:<br />
100% Major depressive disorder by RDC<br />
Exclusions: Intercurrent medical illness, childbearing<br />
potential, and the need to take other medications.<br />
Notes: AMI (15) + PLA (18) = 33 participants. Amitriptyline<br />
(12M:3F) and Placebo (10M:8F).<br />
Baseline: Zimelidine Amitriptyline Placebo<br />
HAM-D 21 (SE) 29.9 (1.1) 28.5 (1.5) 28.6 (1.3)<br />
n= 58<br />
Age: Mean 65 Range 55-76<br />
Sex: 22 males 36 females<br />
Diagnosis:<br />
100% Major depressive disorder by RDC<br />
Exclusions: HAMD-21 < 16; moderate or severe dementia;<br />
drug/alcohol dependence; mental retardation; serious<br />
neurological disorders; other pre-existing major psychiatric<br />
disorders; serious medical illness; urinary retention; narrowangle<br />
glaucoma; supersensitivity to <strong>TCAs</strong> or MAOIs.<br />
Notes: Ns do not include phenelzine group; No M/F based<br />
on % M/F in ITT sample<br />
Baseline: Placebo Nortriptyline Phenelzine<br />
HAM-D 21 23.07 23.58 22.14<br />
n= 60<br />
Age: Mean 68 Range 60-90<br />
Sex: 23 males 37 females<br />
Diagnosis:<br />
100% Depression by RDC<br />
Data Used<br />
Leaving treatment early <strong>for</strong> any reason<br />
Leaving treatment early due to side effects<br />
Data Used<br />
Leaving treatment early <strong>for</strong> any reason<br />
BDI mean endpoint<br />
HRSD-21 mean endpoint<br />
Data Used<br />
Non-remission HRSD-21 < 10<br />
Number reporting side effects<br />
Leaving treatment early <strong>for</strong> any reason<br />
Data Not Used<br />
HRSD-21 mean endpoint - no variablility<br />
measure<br />
Notes: Remission reported as 'response' but<br />
definition closer to that <strong>for</strong> remission on other<br />
studies<br />
Data Used<br />
Non-remission HRSD-17 < 10<br />
Non-response 50% reduction in HRSD<br />
Leaving treatment early <strong>for</strong> any reason<br />
Data Not Used<br />
HRSD-17 mean endpoint - no data<br />
Group<br />
Group<br />
Group<br />
Group<br />
Group<br />
Group<br />
Group<br />
Group<br />
1 N= 22<br />
Imipramine - 2.5mg/kg/day. By study day<br />
9 participants were to achieve a target<br />
dose of 2.5mg/kg/day in three divided<br />
doses. They were to take this <strong>for</strong> 4 weeks.<br />
2 N= 22<br />
Placebo - No details.<br />
1 N= 18<br />
Placebo. Mean dose 223mg/day - No<br />
details.<br />
2 N= 15<br />
Amitriptyline. Mean dose 206mg/day -<br />
150mg/day by the end of week 1 and<br />
300mg/day by the end of week 2.<br />
1 N= 28<br />
Nortriptyline. Mean dose 79 mg/day - 1-3:<br />
25mg/day, then days 4-7: 50mg/day. At<br />
then end of the first week, the daily dose<br />
was increased to 75mg/day. Patients who<br />
attained a plasma level between 50-<br />
180ng/ml at the end of week 2 remained<br />
on 75mg/day. Otherwise, patients took up<br />
to 125mg/day.<br />
2 N= 30<br />
Placebo - Days 1-3: 1 capsules/day, then<br />
days 4-7: 2 capsules/day. At then end of<br />
the first week of treatment, the daily dose<br />
was increased to 3 capsules/day.<br />
1 N= 20<br />
Imipramine. Mean dose 145mg/day - 50-<br />
200mg/day.<br />
2 N= 20<br />
Placebo - Equivalent of 50-200mg/day.<br />
Funding; unclear.<br />
Funding; unknown.<br />
SIGN 1+; funding partly<br />
NIMH grant, no further<br />
details<br />
Funding; part-pharma (Mead<br />
Johnson Pharmaceuticals).<br />
33