TCAs versus placebo - National Center for Biotechnology Information
TCAs versus placebo - National Center for Biotechnology Information
TCAs versus placebo - National Center for Biotechnology Information
Create successful ePaper yourself
Turn your PDF publications into a flip-book with our unique Google optimized e-Paper software.
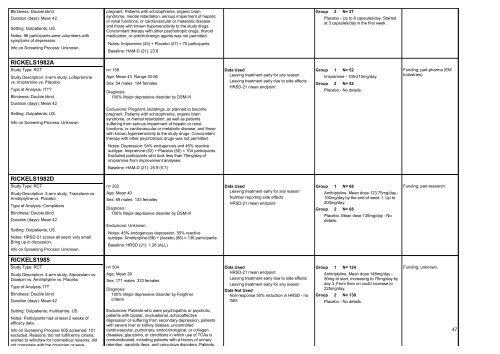
Blindness: Double blind<br />
Duration (days): Mean 42<br />
Setting: Outpatients; US.<br />
Notes: 96 participants were volunteers with<br />
symptoms of depression.<br />
Info on Screening Process: Unknown.<br />
RICKELS1982A<br />
Study Type: RCT<br />
Study Description: 3-arm study; Lofepramine<br />
vs. Imipramine vs. Placebo<br />
Type of Analysis: ITT?<br />
Blindness: Double blind<br />
Duration (days): Mean 42<br />
Setting: Outpatients; US.<br />
Info on Screening Process: Unknown.<br />
RICKELS1982D<br />
Study Type: RCT<br />
Study Description: 3-arm study; Trazodone vs.<br />
Amitriptyline vs. Placebo<br />
Type of Analysis: Completers<br />
Blindness: Double blind<br />
Duration (days): Mean 42<br />
Setting: Outpatients; US.<br />
Notes: HRSD-21 scores all seem very small.<br />
Bring up in discussion.<br />
Info on Screening Process: Unknown.<br />
RICKELS1985<br />
Study Type: RCT<br />
Study Description: 4-arm study; Alprazolam vs.<br />
Doxepin vs. Amitriptyline vs. Placebo<br />
Type of Analysis: ITT<br />
Blindness: Double blind<br />
Duration (days): Mean 42<br />
Setting: Outpatients; multicentre, US.<br />
Notes: Participants had at least 2 weeks of<br />
efficacy data.<br />
Info on Screening Process: 605 screened; 101<br />
excluded. Reasons; did not fulfill entry criteria,<br />
wished to withdraw <strong>for</strong> nonmedical reasons, did<br />
not cooperate with the physician or were<br />
pregnant. Patients with schizophrenia, organic brain<br />
syndrome, mental retardation, serious impairment of hepatic<br />
or renal functions, or cardiovascular or metabolic disease<br />
and those with known hypersensitivity to the study drugs.<br />
Concomitant therapy with other psychotropic drugs, thyroid<br />
medication, or anticholinergic agents was not permitted.<br />
Notes: Imipramine (43) + Placebo (27) = 70 participants.<br />
Baseline: HAM-D (21): 23.8<br />
n= 158<br />
Age: Mean 43 Range 30-56<br />
Sex: 54 males 104 females<br />
Diagnosis:<br />
100% Major depressive disorder by DSM-III<br />
Exclusions: Pregnant, lactatings, or planned to become<br />
pregnant. Patients with schizophrenia, organic brain<br />
syndrome, or mental retardation, as well as patients<br />
suffering from serious impairment of hepatic or renal<br />
functions, or cardiovascular or metabolic disease, and those<br />
with known hypersensitivity to the study drugs. Concomitant<br />
therapy with other psychotropic drugs was not permitted.<br />
Notes: Depression: 54% endogenous and 46% reactive<br />
subtype. Imipramine (52) + Placebo (52) = 104 participants.<br />
Excluded participants who took less than 75mg/day of<br />
imipramine from improvement analyses.<br />
Baseline: HAM-D (21): 25.9 (5.7)<br />
n= 202<br />
Age: Mean 40<br />
Sex: 69 males 133 females<br />
Diagnosis:<br />
100% Major depressive disorder by DSM-III<br />
Exclusions: Unknown.<br />
Notes: 45% endogenous depression. 55% reactive<br />
subtype. Amitriptyline (68) + <strong>placebo</strong> (68) = 136 participants.<br />
Baseline: HRSD (21): 1.26 (ALL)<br />
n= 504<br />
Age: Mean 39<br />
Sex: 171 males 333 females<br />
Diagnosis:<br />
100% Major depressive disorder by Feighner<br />
criteria<br />
Exclusions: Patients who were psychopathic or psychotic,<br />
patients with bipolar, involuational, schizoaffective<br />
depression or suffering from secondary depression, patients<br />
with severe liver or kidney disease, uncontrolled<br />
cardiovascular, pulmonary, endocrinological, or collagen<br />
diseases, glaucoma, or conditions in which use of <strong>TCAs</strong> is<br />
contraindicated, including patients with a history of urinary<br />
retention, paralytic ileus, and convulsive disorders. Patients<br />
Data Used<br />
Leaving treatment early <strong>for</strong> any reason<br />
Leaving treatment early due to side effects<br />
HRSD-21 mean endpoint<br />
Data Used<br />
Leaving treatment early <strong>for</strong> any reason<br />
Number reporting side effects<br />
HRSD-21 mean endpoint<br />
Data Used<br />
HRSD-21 mean endpoint<br />
Leaving treatment early due to side effects<br />
Leaving treatment early <strong>for</strong> any reason<br />
Data Not Used<br />
Non-response 50% reduction in HRSD - no<br />
data<br />
Group<br />
Group<br />
Group<br />
Group<br />
Group<br />
Group<br />
Group<br />
2 N= 27<br />
Placebo - Up to 8 capsules/day. Started<br />
at 3 capsules/day in the first week.<br />
1 N= 52<br />
Imipramine - 105-210mg/day.<br />
2 N= 52<br />
Placebo - No details.<br />
1 N= 68<br />
Amitriptyline. Mean dose 123.75mg/day -<br />
100mg/day by the end of week 1. Up to<br />
200mg/day.<br />
2 N= 68<br />
Placebo. Mean dose 135mg/day - No<br />
details.<br />
1 N= 124<br />
Amitriptyline. Mean dose 148mg/day -<br />
50mg to start, increasing to 75mg/day by<br />
day 3. From then on could increase to<br />
225mg/day.<br />
2 N= 130<br />
Placebo - No details.<br />
Funding; part-pharma (EM<br />
Industries).<br />
Funding; part-research.<br />
Funding; unknown.<br />
47