Annual Report 2011 / 2012 - E21 - Technische Universität München
Annual Report 2011 / 2012 - E21 - Technische Universität München
Annual Report 2011 / 2012 - E21 - Technische Universität München
Create successful ePaper yourself
Turn your PDF publications into a flip-book with our unique Google optimized e-Paper software.
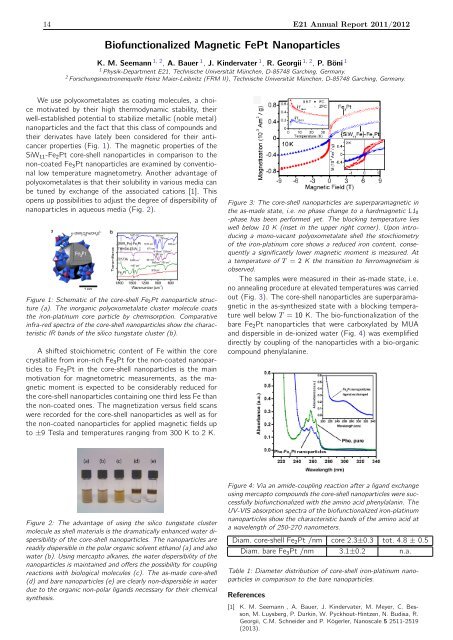
14 <strong>E21</strong> <strong>Annual</strong> <strong>Report</strong> <strong>2011</strong>/<strong>2012</strong><br />
Biofunctionalized Magnetic FePt Nanoparticles<br />
K. M. Seemann 1, 2 , A. Bauer 1 , J. Kindervater 1 , R. Georgii 1, 2 , P. Böni 1<br />
1 Physik-Department <strong>E21</strong>, <strong>Technische</strong> Universität München, D-85748 Garching, Germany.<br />
2 Forschungsneutronenquelle Heinz Maier-Leibnitz (FRM II), <strong>Technische</strong> Universität München, D-85748 Garching, Germany.<br />
We use polyoxometalates as coating molecules, a choice<br />
motivated by their high thermodynamic stability, their<br />
well-established potential to stabilize metallic (noble metal)<br />
nanoparticles and the fact that this class of compounds and<br />
their derivates have lately been considered for their anticancer<br />
properties (Fig. 1). The magnetic properties of the<br />
SiW 11 -Fe 2 Pt core-shell nanoparticles in comparison to the<br />
non-coated Fe 3 Pt nanoparticles are examined by conventional<br />
low temperature magnetometry. Another advantage of<br />
polyoxometalates is that their solubility in various media can<br />
be tuned by exchange of the associated cations [1]. This<br />
opens up possibilities to adjust the degree of dispersibility of<br />
nanoparticles in aqueous media (Fig. 2).<br />
Figure 1: Schematic of the core-shell Fe 2Pt nanoparticle structure<br />
(a). The inorganic polyoxometalate cluster molecule coats<br />
the iron-platinum core particle by chemisorption. Comparative<br />
infra-red spectra of the core-shell nanoparticles show the characteristic<br />
IR bands of the silico tungstate cluster (b).<br />
A shifted stoichiometric content of Fe within the core<br />
crystallite from iron-rich Fe 3 Pt for the non-coated nanoparticles<br />
to Fe 2 Pt in the core-shell nanoparticles is the main<br />
motivation for magnetometric measurements, as the magnetic<br />
moment is expected to be considerably reduced for<br />
the core-shell nanoparticles containing one third less Fe than<br />
the non-coated ones. The magnetization versus field scans<br />
were recorded for the core-shell nanoparticles as well as for<br />
the non-coated nanoparticles for applied magnetic fields up<br />
to ±9 Tesla and temperatures ranging from 300 K to 2 K.<br />
Figure 3: The core-shell nanoparticles are superparamagnetic in<br />
the as-made state, i.e. no phase change to a hardmagnetic L1 0<br />
-phase has been performed yet. The blocking temperature lies<br />
well below 10 K (inset in the upper right corner). Upon introducing<br />
a mono-vacant polyoxometalate shell the stoichiometry<br />
of the iron-platinum core shows a reduced iron content, consequently<br />
a significantly lower magnetic moment is measured. At<br />
a temperature of T = 2 K the transition to ferromagnetism is<br />
observed.<br />
The samples were measured in their as-made state, i.e.<br />
no annealing procedure at elevated temperatures was carried<br />
out (Fig. 3). The core-shell nanoparticles are superparamagnetic<br />
in the as-synthesized state with a blocking temperature<br />
well below T = 10 K. The bio-functionalization of the<br />
bare Fe 2 Pt nanoparticles that were carboxylated by MUA<br />
and dispersible in de-ionized water (Fig. 4) was exemplified<br />
directly by coupling of the nanoparticles with a bio-organic<br />
compound phenylalanine.<br />
Figure 2: The advantage of using the silico tungstate cluster<br />
molecule as shell materials is the dramatically enhanced water dispersibility<br />
of the core-shell nanoparticles. The nanoparticles are<br />
readily dispersible in the polar organic solvent ethanol (a) and also<br />
water (b). Using mercapto alkanes, the water dispersibility of the<br />
nanoparticles is maintained and offers the possibility for coupling<br />
reactions with biological molecules (c). The as-made core-shell<br />
(d) and bare nanoparticles (e) are clearly non-dispersible in water<br />
due to the organic non-polar ligands necessary for their chemical<br />
synthesis.<br />
Figure 4: Via an amide-coupling reaction after a ligand exchange<br />
using mercapto compounds the core-shell nanoparticles were successfully<br />
biofunctionalized with the amino acid phenylalanin. The<br />
UV-VIS absorption spectra of the biofunctionalized iron-platinum<br />
nanoparticles show the characteristic bands of the amino acid at<br />
a wavelength of 250-270 nanometers.<br />
Diam. core-shell Fe 2 Pt /nm core 2.3±0.3 tot. 4.8 ± 0.5<br />
Diam. bare Fe 3 Pt /nm 3.1±0.2 n.a.<br />
Table 1: Diameter distribution of core-shell iron-platinum nanoparticles<br />
in comparison to the bare nanoparticles.<br />
References<br />
[1] K. M. Seemann , A. Bauer, J. Kindervater, M. Meyer, C. Besson,<br />
M. Luysberg, P. Durkin, W. Pyckhout-Hintzen, N. Budisa, R.<br />
Georgii, C.M. Schneider and P. Kögerler, Nanoscale 5 2511-2519<br />
(2013).