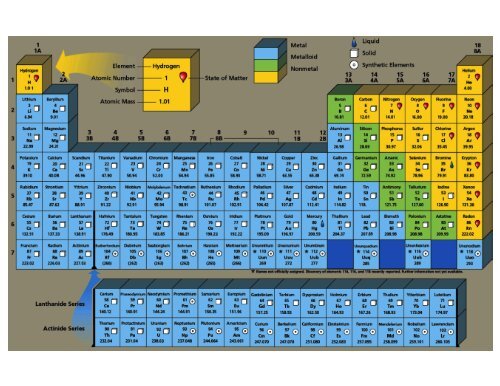
Periodic Table & Common Ion Chart
Periodic Table & Common Ion Chart
Periodic Table & Common Ion Chart
Create successful ePaper yourself
Turn your PDF publications into a flip-book with our unique Google optimized e-Paper software.
Anions Cations<br />
<strong>Common</strong> <strong>Ion</strong>s<br />
+1 Charge +2 Charge +3 Charge<br />
Name Formula Name Formula Name Formula<br />
ammonium NH4 + barium Ba 2+ aluminum Al 3+<br />
copper(I)<br />
Cu<br />
(cuprous)<br />
cadmium Cd 2+ chromium(III)<br />
(chromic)<br />
Cr 3+<br />
hydrogen H + calcium Ca 2+ iron(III)<br />
(ferric)<br />
Fe 3+<br />
lithium Li + chromium(II)<br />
(chromous)<br />
Cr 2+<br />
potassium K + cobalt(II) Co 2+<br />
silver Ag + copper(II)<br />
(cupric)<br />
Cu 2+<br />
sodium Na + iron(II)<br />
(ferrous)<br />
Fe 2+<br />
lead(II)<br />
(plumbous)<br />
Pb 2+<br />
magnesium Mg 2+<br />
manganese(II)<br />
(manganous)<br />
Mn 2+<br />
+4Charge<br />
mercury(I)<br />
Hg2<br />
(mercurous)<br />
lead(IV)<br />
(plumbic)<br />
Pb 4+<br />
mercury(II)<br />
Hg<br />
(mercuric)<br />
tin(IV)<br />
(stannic)<br />
Sn 4+<br />
tin(II)<br />
(stannous)<br />
Sn 2+<br />
zinc Zn 2+<br />
-1 Charge -2 Charge -3 Charge<br />
Name Formula Name Formula Name Formula<br />
acetate<br />
C2H3O2 -<br />
(CH3COO - )<br />
carbonate CO3 2- arsenate AsO4 3-<br />
bromide Br - chromate CrO4 2- nitride N 3-<br />
chloride Cl - dichromate Cr2O7 2- phosphide P 3-<br />
iodide I - phosphate HPO4 2- phosphate PO4 3-<br />
hydrogen<br />
(biphosphate)<br />
hypochlorite ClO - oxalate C2O4 2-<br />
cyanide CN - oxide O 2-<br />
hydrogen<br />
carbonate HCO3 - peroxide O2 2-<br />
(bicarbonate)<br />
chlorate ClO3 - silicate SiO3 2-<br />
fluoride F - sulfate SO4 2-<br />
hydrogen sulfate<br />
(bisulfate)<br />
HSO4 - sulfide S 2-<br />
chlorite ClO2 - sulfite SO3 2-<br />
hydroxide OH - thiosulfate S2O3 2-<br />
nitrate NO3 -<br />
nitrite NO2 -<br />
perchlorate ClO4 -<br />
permanganate MnO4 -