Atom transfer radical polymerizations of styrene and butadiene as ...
Atom transfer radical polymerizations of styrene and butadiene as ...
Atom transfer radical polymerizations of styrene and butadiene as ...
You also want an ePaper? Increase the reach of your titles
YUMPU automatically turns print PDFs into web optimized ePapers that Google loves.
<strong>Atom</strong> <strong>transfer</strong> <strong>radical</strong> <strong>polymerizations</strong> <strong>of</strong> <strong>styrene</strong> <strong>and</strong> <strong>butadiene</strong> <strong>as</strong> well <strong>as</strong> their copolymerization 43<br />
the rate <strong>of</strong> reversible oxidation <strong>and</strong> reduction <strong>of</strong> MoCl 3<br />
(OC 8 H 17 ) 2 <strong>and</strong> the rate constant <strong>of</strong> polymerization with<br />
polymerization temperature.<br />
Figure 3 shows the 1 H NMR spectrum <strong>of</strong> the PSt<br />
obtained with this catalyst system. The signal at δ 4.56 ppm<br />
arises from the terminal proton adjacent to the chlorine<br />
atom in the ω-end group, i.e., –CH 2 C–(C 6 H 5 )H–Cl. This<br />
further verified that the mechanism <strong>of</strong> the synthesis <strong>of</strong> the<br />
poly<strong>styrene</strong> had ATRP character. A chloride atom w<strong>as</strong><br />
<strong>transfer</strong>red between the polymer <strong>radical</strong> <strong>and</strong> the dormant<br />
species.<br />
Butadiene polymerization<br />
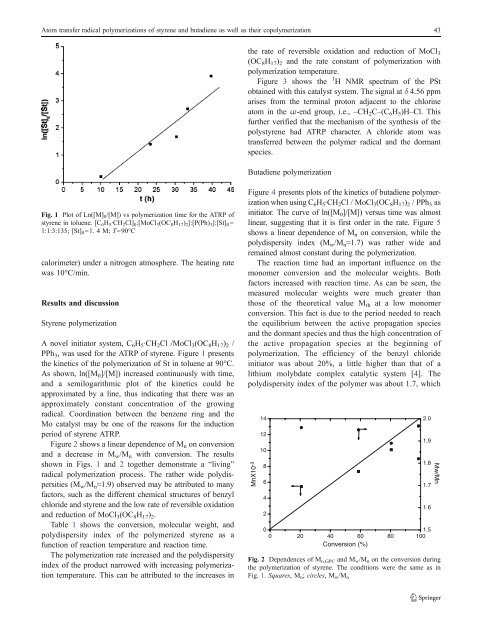
Fig. 1 Plot <strong>of</strong> Ln([M] 0 /[M]) vs polymerization time for the ATRP <strong>of</strong><br />
<strong>styrene</strong> in toluene. [C 6 H 5·CH 2 Cl] 0 :[MoCl 3 (OC 8 H 17 ) 2 ]:[P(Ph) 3 ]:[St] 0 =<br />
1:1:3:135; [St] 0 =1. 4 M; T=90°C<br />
calorimeter) under a nitrogen atmosphere. The heating rate<br />
w<strong>as</strong> 10°C/min.<br />
Results <strong>and</strong> discussion<br />
Styrene polymerization<br />
A novel initiator system, C 6 H 5·CH 2 Cl /MoCl 3 (OC 8 H 17 ) 2 /<br />
PPh 3 , w<strong>as</strong> used for the ATRP <strong>of</strong> <strong>styrene</strong>. Figure 1 presents<br />
the kinetics <strong>of</strong> the polymerization <strong>of</strong> St in toluene at 90°C.<br />
As shown, ln([M 0 ]/[M]) incre<strong>as</strong>ed continuously with time,<br />
<strong>and</strong> a semilogarithmic plot <strong>of</strong> the kinetics could be<br />
approximated by a line, thus indicating that there w<strong>as</strong> an<br />
approximately constant concentration <strong>of</strong> the growing<br />
<strong>radical</strong>. Coordination between the benzene ring <strong>and</strong> the<br />
Mo catalyst may be one <strong>of</strong> the re<strong>as</strong>ons for the induction<br />
period <strong>of</strong> <strong>styrene</strong> ATRP.<br />
Figure 2 shows a linear dependence <strong>of</strong> M n on conversion<br />
<strong>and</strong> a decre<strong>as</strong>e in M w /M n with conversion. The results<br />
shown in Figs. 1 <strong>and</strong> 2 together demonstrate a “living”<br />
<strong>radical</strong> polymerization process. The rather wide polydispersities<br />
(M w /M n ≈1.9) observed may be attributed to many<br />
factors, such <strong>as</strong> the different chemical structures <strong>of</strong> benzyl<br />
chloride <strong>and</strong> <strong>styrene</strong> <strong>and</strong> the low rate <strong>of</strong> reversible oxidation<br />
<strong>and</strong> reduction <strong>of</strong> MoCl 3 (OC 8 H 17 ) 2 .<br />
Table 1 shows the conversion, molecular weight, <strong>and</strong><br />
polydispersity index <strong>of</strong> the polymerized <strong>styrene</strong> <strong>as</strong> a<br />
function <strong>of</strong> reaction temperature <strong>and</strong> reaction time.<br />
The polymerization rate incre<strong>as</strong>ed <strong>and</strong> the polydispersity<br />
index <strong>of</strong> the product narrowed with incre<strong>as</strong>ing polymerization<br />
temperature. This can be attributed to the incre<strong>as</strong>es in<br />
Figure 4 presents plots <strong>of</strong> the kinetics <strong>of</strong> <strong>butadiene</strong> polymerization<br />
when using C 6 H 5·CH 2 Cl / MoCl 3 (OC 8 H 17 ) 2 / PPh 3 <strong>as</strong><br />
initiator. The curve <strong>of</strong> ln([M 0 ]/[M]) versus time w<strong>as</strong> almost<br />
linear, suggesting that it is first order in the rate. Figure 5<br />
shows a linear dependence <strong>of</strong> M n on conversion, while the<br />
polydispersity index (M w /M n ≈1.7) w<strong>as</strong> rather wide <strong>and</strong><br />
remained almost constant during the polymerization.<br />
The reaction time had an important influence on the<br />
monomer conversion <strong>and</strong> the molecular weights. Both<br />
factors incre<strong>as</strong>ed with reaction time. As can be seen, the<br />
me<strong>as</strong>ured molecular weights were much greater than<br />
those <strong>of</strong> the theoretical value M th at a low monomer<br />
conversion. This fact is due to the period needed to reach<br />
the equilibrium between the active propagation species<br />
<strong>and</strong> the dormant species <strong>and</strong> thus the high concentration <strong>of</strong><br />
the active propagation species at the beginning <strong>of</strong><br />
polymerization. The efficiency <strong>of</strong> the benzyl chloride<br />
initiator w<strong>as</strong> about 20%, a little higher than that <strong>of</strong> a<br />
lithium molybdate complex catalytic system [4]. The<br />
polydispersity index <strong>of</strong> the polymer w<strong>as</strong> about 1.7, which<br />
MnX10 -3<br />
14<br />
12<br />
10<br />
8<br />
6<br />
4<br />
2<br />
0<br />
1.5<br />
0 20 40 60 80 100<br />
Conversion (%)<br />
Fig. 2 Dependences <strong>of</strong> M n,GPC <strong>and</strong> M w /M n on the conversion during<br />
the polymerization <strong>of</strong> <strong>styrene</strong>. The conditions were the same <strong>as</strong> in<br />
Fig. 1. Squares, M n ; circles, M w /M n<br />
2.0<br />
1.9<br />
1.8<br />
1.7<br />
1.6<br />
Mw/Mn