You also want an ePaper? Increase the reach of your titles
YUMPU automatically turns print PDFs into web optimized ePapers that Google loves.
10<br />
HOMEWORK!!!<br />
Complete TABLE E in your booklet for homework!<br />
Do a few examples in class so that when you go home, you know what you are doing!!!<br />
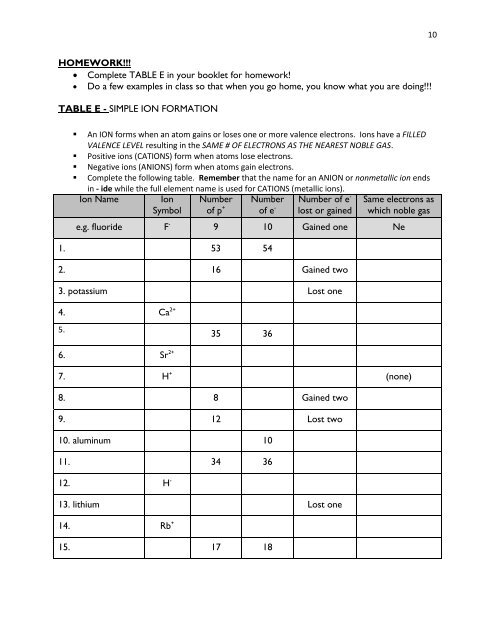
TABLE E - SIMPLE ION <strong>FOR</strong>MATION<br />
• An ION forms when an atom gains or loses one or more valence electrons. Ions have a FILLED<br />
VALENCE LEVEL resulting in the SAME # OF ELECTRONS AS THE NEAREST NOBLE GAS.<br />
• Positive ions (CATIONS) form when atoms lose electrons.<br />
• Negative ions (ANIONS) form when atoms gain electrons.<br />
• Complete the following table. Remember that the name for an ANION or nonmetallic ion ends<br />
in - ide while the full element name is used for CATIONS (metallic ions).<br />
Ion Name<br />
Ion<br />
Symbol<br />
Number<br />
of p +<br />
Number<br />
of e - Number of e -<br />
lost or gained<br />
Same electrons as<br />
which noble gas<br />
e.g. fluoride F - 9 10 Gained one Ne<br />
1. 53 54<br />
2. 16 Gained two<br />
3. potassium Lost one<br />
4. Ca 2+<br />
5.<br />
35 36<br />
6. Sr 2+<br />
7. H + (none)<br />
8. 8 Gained two<br />
9. 12 Lost two<br />
10. aluminum 10<br />
11. 34 36<br />
12. H -<br />
13. lithium Lost one<br />
14. Rb +<br />
15. 17 18