Organic Chemistry Structures of Organic Compounds
Organic Chemistry Structures of Organic Compounds
Organic Chemistry Structures of Organic Compounds
You also want an ePaper? Increase the reach of your titles
YUMPU automatically turns print PDFs into web optimized ePapers that Google loves.
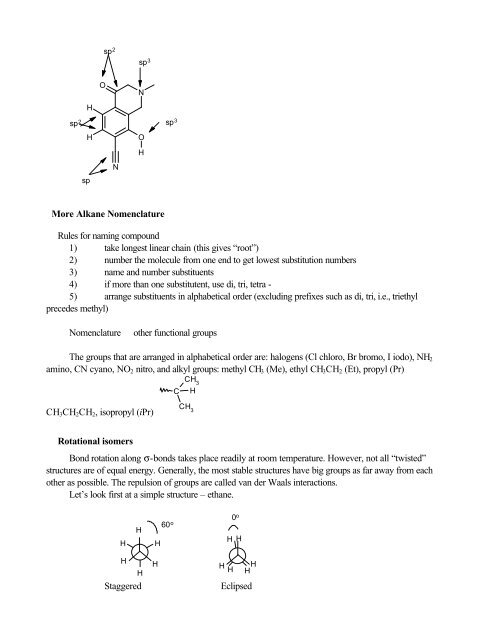
sp 2 sp 3 sp 3HONsp 2HspNOHMore Alkane NomenclatureRules for naming compound1) take longest linear chain (this gives “root”)2) number the molecule from one end to get lowest substitution numbers3) name and number substituents4) if more than one substitutent, use di, tri, tetra -5) arrange substituents in alphabetical order (excluding prefixes such as di, tri, i.e., triethylprecedes methyl)Nomenclatureother functional groupsThe groups that are arranged in alphabetical order are: halogens (Cl chloro, Br bromo, I iodo), NH 2amino, CN cyano, NO 2 nitro, and alkyl groups: methyl CH 3 (Me), ethyl CH 3 CH 2 (Et), propyl (Pr)CCH 3HCH 3 CH 2 CH 2 , isopropyl (iPr)CH 3Rotational isomersBond rotation along σ-bonds takes place readily at room temperature. However, not all “twisted”structures are <strong>of</strong> equal energy. Generally, the most stable structures have big groups as far away from eachother as possible. The repulsion <strong>of</strong> groups are called van der Waals interactions.Let’s look first at a simple structure – ethane.HH0 o60 o H HHHHStaggeredHH HH HEclipsed