Blood glucose management in type 2 diabetes - NHS Cumbria
Blood glucose management in type 2 diabetes - NHS Cumbria
Blood glucose management in type 2 diabetes - NHS Cumbria
You also want an ePaper? Increase the reach of your titles
YUMPU automatically turns print PDFs into web optimized ePapers that Google loves.
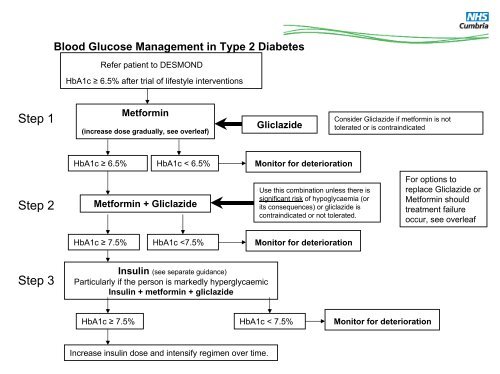
<strong>Blood</strong> Glucose Management <strong>in</strong> Type 2 DiabetesRefer patient to DESMONDHbA1c ≥ 6.5% after trial of lifestyle <strong>in</strong>terventionsStep 1Metform<strong>in</strong>(<strong>in</strong>crease dose gradually, see overleaf)GliclazideConsider Gliclazide if metform<strong>in</strong> is nottolerated or is contra<strong>in</strong>dicatedHbA1c ≥ 6.5%HbA1c < 6.5%Monitor for deteriorationStep 2Metform<strong>in</strong> + GliclazideUse this comb<strong>in</strong>ation unless there issignificant risk of hypoglycaemia (orits consequences) or gliclazide iscontra<strong>in</strong>dicated or not tolerated.For options toreplace Gliclazide orMetform<strong>in</strong> shouldtreatment failureoccur, see overleafHbA1c ≥ 7.5%HbA1c
All newly diagnosed Type 2 diabetics should be referred to DESMOND.Diet is the usual <strong>in</strong>itial therapy follow<strong>in</strong>g diagnosis of <strong>type</strong> 2 <strong>diabetes</strong>,Drug therapy should be considered earlier for patients with marked symptoms and significant hyperglycaemia.Patients must <strong>in</strong>form DVLA / <strong>in</strong>surance company when commenc<strong>in</strong>g oral hypoglycaemic drugs.UKPDS demonstrated that tight control of blood <strong>glucose</strong> average HbA1c of 7.0% versus 7.9% is associated with a reduced risk ofmicrovascular complications.1st Choice Metform<strong>in</strong>Metform<strong>in</strong> is the only oral antidiabetic drug which has a proven survival advantage <strong>in</strong> overweight patients (BMI > 25) and gavegreater risk reductions than other therapies for both microvascular and macrovascular complications, with less weight ga<strong>in</strong>.(UKPDS). Hypoglycaemia does not occur with Metform<strong>in</strong> therapy.Metform<strong>in</strong> is safe to use <strong>in</strong> the non-obese and shows the greatest reduction <strong>in</strong> HbA1c of all therapies. Step up Metform<strong>in</strong> every 10 –15 days over several weeks to m<strong>in</strong>imise risk of gastro<strong>in</strong>test<strong>in</strong>al (GI) side effects.Renal function should be monitored, and the appropriateness of the dose and drug re-assessed if renal function deteriorates.Consider trial of extended-absorption Metform<strong>in</strong> if GI <strong>in</strong>tolerability. Convert back to standard tablets after 3 months2nd Choice GliclazideEducate the patient about the possible risk of hypoglycaemia, particularly if he or she has renal impairment.Gliclazide should be taken before mealsOptions after poor control (HbA1c >7.5) with Metform<strong>in</strong> and Gliclazidea) Insul<strong>in</strong>• Human Isophane Insul<strong>in</strong> should be offered <strong>in</strong>itially. See <strong>in</strong>sul<strong>in</strong> guidance.b) Sitaglipt<strong>in</strong>, Vildaglipt<strong>in</strong>● Cont<strong>in</strong>ue treatment with Sitaglipt<strong>in</strong> or Vildaglipt<strong>in</strong> only if there is a reduction of ≥ 0.5 percentage po<strong>in</strong>ts <strong>in</strong> HbA1c <strong>in</strong> 6 months.They have been shown to reduce HbA1c levels, but, currently there is no data on morbidity, mortality or long-term adverse effectsc) Pioglitazone● Cont<strong>in</strong>ue treatment with pioglitazone therapy only if there is a reduction of ≥ 0.5 percentage po<strong>in</strong>ts <strong>in</strong> HbA1c <strong>in</strong> 6 months.● Glitazones can cause weight ga<strong>in</strong> and fluid retention and reduce bone m<strong>in</strong>eral density <strong>in</strong> post-menopausal women Do not start orcont<strong>in</strong>ue therapy if the person has heart failure or is at higher risk of fracture.● Requires liver function monitor<strong>in</strong>gd) Exenatide (Specialist Referral - Patients BMI>35)● Cont<strong>in</strong>ue exenatide only if there is a reduction <strong>in</strong> HbA1c of ≥ 1.0 % and ≥ 3% of <strong>in</strong>itial body weight <strong>in</strong> 6 months. Exenatide shouldbe used with caution <strong>in</strong> adults older than 75 years and if eGFR