You also want an ePaper? Increase the reach of your titles
YUMPU automatically turns print PDFs into web optimized ePapers that Google loves.
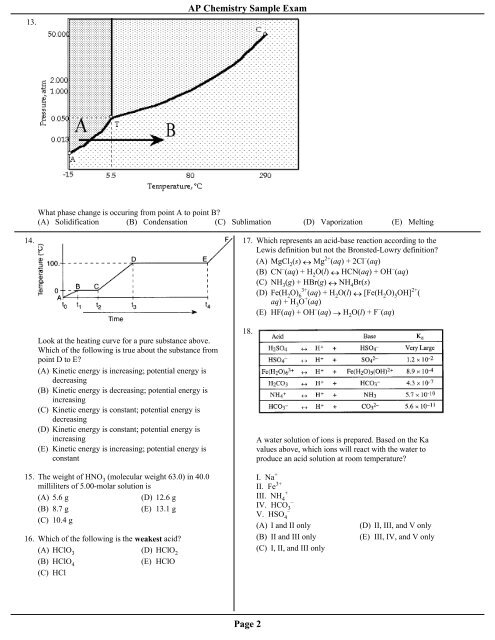
AP Chemistry Sample Exam13.What phase change is occuring from point A to point B?(A) Solidification (B) Condensation (C) Sublimation (D) Vaporization (E) Melting14.17. Which represents an acid-base reaction according to theLewis definition but not the Bronsted-Lowry definition?(A) MgCl 2(s) ↔ Mg 2+ (aq) + 2Cl – (aq)(B) CN – (aq) + H 2O(l) ↔ HCN(aq) + OH – (aq)(C) NH 3(g) + HBr(g) ↔ NH 4Br(s)(D) Fe(H 2O) 6 3+ (aq) + H 2O(l) ↔ [Fe(H 2O) 5OH] 2+ (aq) + H 3O + (aq)(E) HF(aq) + OH – (aq) → H 2O(l) + F – (aq)Look at the heating curve for a pure substance above.Which of the following is true about the substance frompoint D to E?(A) Kinetic energy is increasing; potential energy isdecreasing(B) Kinetic energy is decreasing; potential energy isincreasing(C) Kinetic energy is constant; potential energy isdecreasing(D) Kinetic energy is constant; potential energy isincreasing(E) Kinetic energy is increasing; potential energy isconstant18.A water solution of ions is prepared. Based on the Kavalues above, which ions will react with the water toproduce an acid solution at room temperature?15. <strong>The</strong> weight of HNO 3(molecular weight 63.0) in 40.0milliliters of 5.00-molar solution is(A) 5.6 g(D) 12.6 g(B) 8.7 g(E) 13.1 g(C) 10.4 g16. Which of the following is the weakest acid?(A) HClO 3(D) HClO 2(B) HClO 4(E) HClO(C) HClI. Na +II. Fe 3++III. NH 4–IV. HCO 3–V. HSO 4(A) I and II only(B) II and III only(C) I, II, and III only(D) II, III, and V only(E) III, IV, and V onlyPage 2