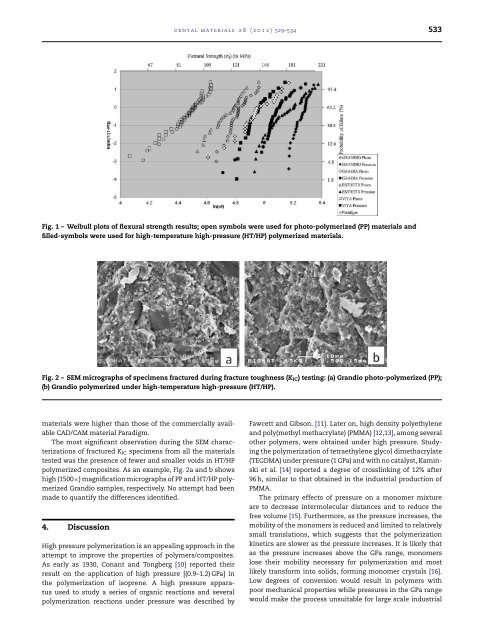
532 d e n t a l m a t e r i a l s 2 8 ( 2 0 1 2 ) 529–534where is density of the sample, A the mass of the samplein air, B the mass of the sample in deionized water, w thedensity of deionized water determined from the measurementof water <strong>temperature</strong>, and a the density of air (0.0012 g/cm 3 ).Thirty determinations on five specimens were made for eachmaterial.2.6. Fractured surface morphologyRepresentative fractured NTP specimens of HT/HP polymerized,Paradigm, and control PP <strong>composite</strong>s were sputtercoated(SC500, Bio-Rad, UK) with gold. The surface morphologywas then characterized under a scanning electronmicroscope (SEM) (JSM-6400, JEOL Ltd., Tokyo, Japan) at lowand <strong>high</strong> magnification.2.7. Statistical analysisSince the main purpose of this study was to assess the effect ofHT/HP polymerization on the physical/mechanical propertiesand not to compare materials among themselves, the resultsof mechanical/physical tests for each material were analyzedby t-tests, performed at a 0.05 level of significance. The resultsobtained for Paradigm were used as a reference only and werenot included in the statistical analysis.Weibull statistics parameters were calculated for the flexuralstrength data. The description of the Weibull distributionis given byP f = 1 − e −(/ 0) mwhere P f is the fracture probability, defined by the relationP f =N + 1where K is the rank in strength from least to greatest, Ndenotes the total number of specimens in the sample, m isthe shape parameter (Weibull modulus), and 0 is the scaleparameter or characteristic strength 63,21% [8,9]. The Weibullmodulus, characteristic strength, and the strength at a probabilityof failure of 5% were obtained using the Weibull statisticsoption in Excel ® (Microsoft, USA).3. ResultsThe results of the physical/mechanical characterizations aresummarized in Table 2, along with the results of the statisticalanalysis. Fig. 1 presents Weibull plots of the flexural strengthresults where, to facilitate observation of the effect of HT/HPpolymerization, open symbols were used for PP <strong>composite</strong>s,corresponding filled symbols for their HT/HP counterparts,and a distinct symbol for Paradigm. The results have shownthat HT/HP polymerization resulted in a significant (p < 0.05)increase in flexural strength, hardness, and density for all<strong>composite</strong>s investigated (Table 2) in comparison with their PPcounterparts. Even if the fracture toughness of all materialswas increased by HT/HP polymerization, significant (p < 0.05)increases were detected only for Gradia and EsthetX. TheWeibull modulus of the HT/HP polymerized <strong>composite</strong>s wasalso <strong>high</strong>er than that of PP counterparts. The results for f , itsassociated Weibull modulus, and K IC obtained for the HT/HPTable 2 – Results (Mean ± SD) of physical/mechanical characterizations and statistical analysis. *PVIL VIP PercentincreaseDEL DEP PercentincreaseVOL VOP PercentincreaseGroup a property GCL GCP PercentincreaseK IC (in MPa·m 1/2 ) 0.85 ± 0.08 1.58 ± 0.18 85.88 * 1.03 ± 0.11 1.17 ± 0.21 13.59 1.26 ± 0.21 1.52 ± 0.10 20.63 * 0.97 ± 0.15 0.99 ± 0.17 2.06 0.78 ± 0.21Hardness (in HVN) 33.01 ± 5.11 53.78 ± 2.26 62.92 * 85.66 ± 5.65 110.02 ± 4.31 28.43 * 38.95 ± 3.48 79.64 ± 2.52 104.46 * 20.01 ± 1.98 40.73 ± 3.16 103.54 * 114.80 ± 4.34Density (in g/cm 3 ) 1.6250 ± 0.0023 1.6337 ± 0.0018 0.53 * 2.1795 ± 0.0017 2.1868 ± 0.0012 0.33 * 2.0754 ± 0.0038 2.1008 ± 0.0068 1.22 * 1.5044 ± 0.0012 1.5087 ± 0.0010 0.29 * 2.1261 ± 0.0042Weibull modulus 7.04 10.34 11.42 22.39 5.62 8.87 4.66 13.88 5.61Identifies significantly different (p < 0.05) results based on pairwise t-test comparisons.a GCL – Gradia light cured; GCP – Gradia HP/HT polymerization; VOL – Grandio light cured; – Vita VM LC light cured; VIP – Vita VM LC HP/HT polymerization; P – Paradigm.
d e n t a l m a t e r i a l s 2 8 ( 2 0 1 2 ) 529–534 533Fig. 1 – Weibull plots of flexural strength results; open symbols were used for photo-polymerized (PP) materials andfilled-symbols were used for <strong>high</strong>-<strong>temperature</strong> <strong>high</strong>-<strong>pressure</strong> (HT/HP) polymerized materials.Fig. 2 – SEM micrographs of specimens fractured during fracture toughness (K IC ) testing: (a) Grandio photo-polymerized (PP);(b) Grandio polymerized under <strong>high</strong>-<strong>temperature</strong> <strong>high</strong>-<strong>pressure</strong> (HT/HP).materials were <strong>high</strong>er than those of the commercially availableCAD/CAM material Paradigm.The most significant observation during the SEM characterizationsof fractured K IC specimens from all the materialstested was the presence of fewer and smaller voids in HT/HPpolymerized <strong>composite</strong>s. As an example, Fig. 2a and b shows<strong>high</strong> (1500×) magnification micrographs of PP and HT/HP polymerizedGrandio samples, respectively. No attempt had beenmade to quantify the differences identified.4. DiscussionHigh <strong>pressure</strong> polymerization is an appealing approach in theattempt to improve the properties of polymers/<strong>composite</strong>s.As early as 1930, Conant and Tongberg [10] reported theirresult on the application of <strong>high</strong> <strong>pressure</strong> [(0.9–1.2) GPa] inthe polymerization of isoprene. A <strong>high</strong> <strong>pressure</strong> apparatusused to study a series of organic reactions and severalpolymerization reactions under <strong>pressure</strong> was described byFawcett and Gibson. [11]. Later on, <strong>high</strong> density polyethyleneand poly(methyl methacrylate) (PMMA) [12,13], among severalother polymers, were obtained under <strong>high</strong> <strong>pressure</strong>. Studyingthe polymerization of tetraethylene glycol dimethacrylate(TEGDMA) under <strong>pressure</strong> (1 GPa) and with no catalyst, Kaminskiet al. [14] reported a degree of crosslinking of 12% after96 h, similar to that obtained in the industrial production ofPMMA.The primary effects of <strong>pressure</strong> on a monomer mixtureare to decrease intermolecular distances and to reduce thefree volume [15]. Furthermore, as the <strong>pressure</strong> increases, themobility of the monomers is reduced and limited to relativelysmall translations, which suggests that the polymerizationkinetics are slower as the <strong>pressure</strong> increases. It is likely thatas the <strong>pressure</strong> increases above the GPa range, monomerslose their mobility necessary for polymerization and mostlikely transform into solids, forming monomer crystals [16].Low degrees of conversion would result in polymers withpoor mechanical properties while <strong>pressure</strong>s in the GPa rangewould make the process unsuitable for large scale industrial