Lecture 10: Geochronology VI: U-Th decay series dating
Lecture 10: Geochronology VI: U-Th decay series dating
Lecture 10: Geochronology VI: U-Th decay series dating
You also want an ePaper? Increase the reach of your titles
YUMPU automatically turns print PDFs into web optimized ePapers that Google loves.
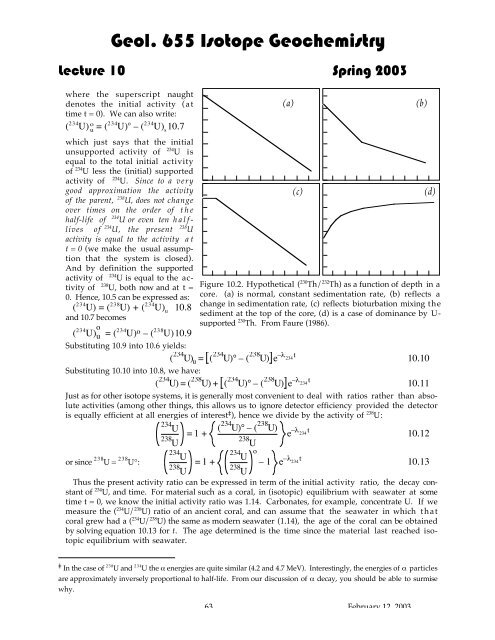
Geol. 655 Isotope Geochemistry<strong>Lecture</strong> <strong>10</strong> Spring 2003where the superscript naughtdenotes the initial activity (attime t = 0). We can also write:( 234 U) uo= ( 234 U) o – ( 234 U) s<strong>10</strong>.7which just says that the initialunsupported activity of 234 U isequal to the total initial activityof 234 U less the (initial) supportedactivity of 234 U. Since to a verygood approximation the activityof the parent, 238 U, does not changeover times on the order of thehalf-life of 234 U or even ten halflivesof 234 U, the present 238 Uactivity is equal to the activity att = 0 (we make the usual assumptionthat the system is closed).And by definition the supportedactivity of 234 U is equal to the activityof 238 U, both now and at t =0. Hence, <strong>10</strong>.5 can be expressed as:( 234 U) = ( 238 U) + ( 234 U) u<strong>10</strong>.8and <strong>10</strong>.7 becomes( 234 U) o u = (234 U) o – ( 238 U)<strong>10</strong>.9Substituting <strong>10</strong>.9 into <strong>10</strong>.6 yields:234 234( U )u = ( U )° – (238 U)e–l 234tSubstituting <strong>10</strong>.<strong>10</strong> into <strong>10</strong>.8, we have:234( U ) = (238 U)+ (234 U)°– (238 U)e–l 234t<strong>10</strong>.<strong>10</strong><strong>10</strong>.11Just as for other isotope systems, it is generally most convenient to deal with ratios rather than absoluteactivities (among other things, this allows us to ignore detector efficiency provided the detectoris equally efficient at all energies of interest ‡ ), hence we divide by the activity of 238 U:234U= 1 + ( 234 U )° – (238 U)e –l 234 t <strong>10</strong>.12238238or since 238 U = 238 U°:UU234= 1 +234U238Uo– 1 e –l 234 t <strong>10</strong>.13238UU<strong>Th</strong>us the present activity ratio can be expressed in term of the initial activity ratio, the <strong>decay</strong> constantof 234 U, and time. For material such as a coral, in (isotopic) equilibrium with seawater at sometime t = 0, we know the initial activity ratio was 1.14. Carbonates, for example, concentrate U. If wemeasure the ( 234 U/ 238 U) ratio of an ancient coral, and can assume that the seawater in which thatcoral grew had a ( 234 U/ 238 U) the same as modern seawater (1.14), the age of the coral can be obtainedby solving equation <strong>10</strong>.13 for t. <strong>Th</strong>e age determined is the time since the material last reached isotopicequilibrium with seawater.(a)(c)Figure <strong>10</strong>.2. Hypothetical ( 230 <strong>Th</strong>/ 232 <strong>Th</strong>) as a function of depth in acore. (a) is normal, constant sedimentation rate, (b) reflects achange in sedimentation rate, (c) reflects bioturbation mixing thesediment at the top of the core, (d) is a case of dominance by U-supported 230 <strong>Th</strong>. From Faure (1986).(b)(d)‡ In the case of 238 U and 234 U the a energies are quite similar (4.2 and 4.7 MeV). Interestingly, the energies of a particlesare approximately inversely proportional to half-life. From our discussion of a <strong>decay</strong>, you should be able to surmisewhy.63 February 12, 2003