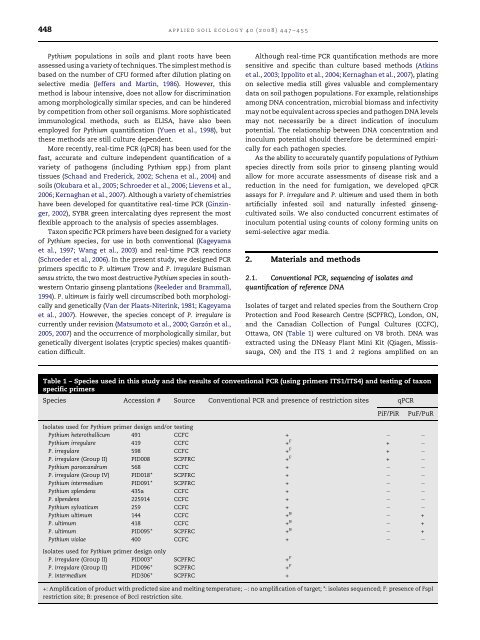
448applied soil ecology 40 (2008) 447–455<strong>Pythium</strong> <strong>populations</strong> <strong>in</strong> <strong>soils</strong> and plant roots have beenassessed us<strong>in</strong>g a variety <strong>of</strong> techniques. The simplest method isbased on the number <strong>of</strong> CFU formed after dilution plat<strong>in</strong>g onselective media (Jeffers and Mart<strong>in</strong>, 1986). However, thismethod is labour <strong>in</strong>tensive, does not allow for discrim<strong>in</strong>ationamong morphologically similar species, and can be h<strong>in</strong>deredby competition from other soil organisms. More sophisticatedimmunological methods, such as ELISA, have also beenemployed for <strong>Pythium</strong> quantification (Yuen et al., 1998), butthese methods are still culture dependent.More recently, real-time PCR (qPCR) has been used for thefast, accurate and culture <strong>in</strong>dependent quantification <strong>of</strong> avariety <strong>of</strong> pathogens (<strong>in</strong>clud<strong>in</strong>g <strong>Pythium</strong> spp.) from planttissues (Schaad and Frederick, 2002; Schena et al., 2004) and<strong>soils</strong> (Okubara et al., 2005; Schroeder et al., 2006; Lievens et al.,2006; Kernaghan et al., 2007). Although a variety <strong>of</strong> chemistrieshave been developed for quantitative real-time PCR (G<strong>in</strong>z<strong>in</strong>ger,2002), SYBR green <strong>in</strong>tercalat<strong>in</strong>g dyes represent the mostflexible approach to the analysis <strong>of</strong> species assemblages.Taxon specific PCR primers have been designed for a variety<strong>of</strong> <strong>Pythium</strong> species, for use <strong>in</strong> both conventional (Kageyamaet al., 1997; Wang et al., 2003) and real-time PCR reactions(Schroeder et al., 2006). In the present study, we designed PCRprimers specific to P. ultimum Trow and P. irregulare Buismansensu stricto, the two most destructive <strong>Pythium</strong> species <strong>in</strong> southwesternOntario g<strong>in</strong>seng plantations (Reeleder and Brammall,1994). P. ultimum is fairly well circumscribed both morphologicallyand genetically (Van der Plaats-Niter<strong>in</strong>k, 1981; Kageyamaet al., 2007). However, the species concept <strong>of</strong> P. irregulare iscurrently under revision (Matsumoto et al., 2000; Garzón etal.,2005, 2007) and the occurrence <strong>of</strong> morphologically similar, butgenetically divergent isolates (cryptic species) makes quantificationdifficult.Although real-time PCR quantification methods are moresensitive and specific than culture based methods (Atk<strong>in</strong>set al., 2003; Ippolito et al., 2004; Kernaghan et al., 2007), plat<strong>in</strong>gon selective media still gives valuable and complementarydata on soil pathogen <strong>populations</strong>. For example, relationshipsamong DNA concentration, microbial biomass and <strong>in</strong>fectivitymay not be equivalent across species and pathogen DNA levelsmay not necessarily be a direct <strong>in</strong>dication <strong>of</strong> <strong>in</strong>oculumpotential. The relationship between DNA concentration and<strong>in</strong>oculum potential should therefore be determ<strong>in</strong>ed empiricallyfor each pathogen species.As the ability to accurately quantify <strong>populations</strong> <strong>of</strong> <strong>Pythium</strong>species directly from <strong>soils</strong> prior to g<strong>in</strong>seng plant<strong>in</strong>g wouldallow for more accurate assessments <strong>of</strong> disease risk and areduction <strong>in</strong> the need for fumigation, we developed qPCRassays for P. irregulare and P. ultimum and used them <strong>in</strong> bothartificially <strong>in</strong>fested soil and naturally <strong>in</strong>fested g<strong>in</strong>sengcultivated<strong>soils</strong>. We also conducted concurrent estimates <strong>of</strong><strong>in</strong>oculum potential us<strong>in</strong>g counts <strong>of</strong> colony form<strong>in</strong>g units onsemi-selective agar media.2. Materials and methods2.1. Conventional PCR, sequenc<strong>in</strong>g <strong>of</strong> isolates andquantification <strong>of</strong> reference DNAIsolates <strong>of</strong> target and related species from the Southern CropProtection and Food Research Centre (SCPFRC), London, ON,and the Canadian Collection <strong>of</strong> Fungal Cultures (CCFC),Ottawa, ON (Table 1) were cultured on V8 broth. DNA wasextracted us<strong>in</strong>g the DNeasy Plant M<strong>in</strong>i Kit (Qiagen, Mississauga,ON) and the ITS 1 and 2 regions amplified on anTable 1 – Species used <strong>in</strong> this study and the results <strong>of</strong> conventional PCR (us<strong>in</strong>g primers ITS1/ITS4) and test<strong>in</strong>g <strong>of</strong> taxonspecific primersSpecies Accession # Source Conventional PCR and presence <strong>of</strong> restriction sites qPCRPiF/PiRPuF/PuRIsolates used for <strong>Pythium</strong> primer design and/or test<strong>in</strong>g<strong>Pythium</strong> heterothallicum 491 CCFC +<strong>Pythium</strong> irregulare 419 CCFC + F +P. irregulare 598 CCFC + F +P. irregulare (Group II) PID008 SCPFRC + F +<strong>Pythium</strong> paroecandrum 568 CCFC +P. irregulare (Group IV) PID018* SCPFRC +<strong>Pythium</strong> <strong>in</strong>termedium PID091* SCPFRC +<strong>Pythium</strong> splendens 435a CCFC +P. slpendens 225914 CCFC +<strong>Pythium</strong> sylvaticum 259 CCFC +<strong>Pythium</strong> ultimum 144 CCFC + B +P. ultimum 418 CCFC + B +P. ultimum PID095* SCPFRC + B +<strong>Pythium</strong> violae 400 CCFC +Isolates used for <strong>Pythium</strong> primer design onlyP. irregulare (Group II) PID003* SCPFRC + FP. irregulare (Group II) PID096* SCPFRC + FP. <strong>in</strong>termedium PID306* SCPFRC ++: Amplification <strong>of</strong> product with predicted size and melt<strong>in</strong>g temperature; : no amplification <strong>of</strong> target; *: isolates sequenced; F: presence <strong>of</strong> FspIrestriction site; B: presence <strong>of</strong> BccI restriction site.
applied soil ecology 40 (2008) 447–455 449Eppendorf Mastercycler (Br<strong>in</strong>kmann Instruments, Mississauga,ON) thermocycler <strong>in</strong> 50 mL reactions conta<strong>in</strong><strong>in</strong>g 1 UPlat<strong>in</strong>um 1 Taq DNA Polymerase, 1 PCR buffer, 200 mM dNTPmix (all from Invitrogen, Burl<strong>in</strong>gton, ON), 2.5 mM MgCl 2 , 0.8%BSA, 0.4 mM each <strong>of</strong> primers ITS 1 and ITS 4 (White et al., 1990)and 5 mL template DNA. Cycl<strong>in</strong>g parameters were 94 8C for2 m<strong>in</strong> followed by 35 cycles <strong>of</strong> 94 8C for 1 m<strong>in</strong>, 55 8C for 1 m<strong>in</strong>and 72 8C for 2 m<strong>in</strong>, followed by 72 8C for 10 m<strong>in</strong>.PCR products generated from <strong>Pythium</strong> isolates from southwesternOntario g<strong>in</strong>seng <strong>soils</strong> (PID003, PID018, PID091,PID095, PID096, PID306) (Table 1) were purified with theM<strong>in</strong>Elute PCR Purification Kit (Qiagen, Mississauga, ON) andsequenced on an ABI 377 Sequencer (Applied Biosystems,Foster City, CA) us<strong>in</strong>g BigDye 1 Term<strong>in</strong>ator chemistry(Applied Biosystems); Genbank accession numbersDQ083527–DQ083532.Reference DNA was obta<strong>in</strong>ed from fresh mycelium <strong>of</strong> P.irregulare (PID 008) and P. ultimum (PID 095) (Table 1) byextract<strong>in</strong>g with the DNeasy Plant M<strong>in</strong>i Kit (Qiagen) andquantify<strong>in</strong>g with an Eppendorf BioPhotometer calibratedwith calf thymus DNA (Sigma) <strong>in</strong> Qiagen elution buffer(buffer AE).2.2. Design <strong>of</strong> taxon specific primers and identification <strong>of</strong>specific restriction sitesTaxon specific primer pairs target<strong>in</strong>g the <strong>in</strong>ternallytranscribed spacer regions <strong>of</strong> P. irregulare Groups I and II(sensu Matsumoto et al., 2000) (PiF 5 0 -GTAGCATGCGT-GTTTGCTTA-3 0 /PiR 5 0 -GCAAGCTGTGCATTCATTGC-3 0 ) andP. ultimum (PuF 5 0 -ATGATGGACTAGCTGATGAA-3 0 /PuR 5 0 -TTCCATTACACTTCATAGAA-3 0 )weredesignedonthebasis<strong>of</strong> an alignment <strong>of</strong> sequenced SCPFRC and CCFC <strong>Pythium</strong>cultures and over 100 <strong>Pythium</strong> sequences from Genbankus<strong>in</strong>g BioEdit ver. 7.0.0 (Hall, 1999). The predicted PCRproduct sizes were 508 and 407 bp, respectively. The extent<strong>of</strong> primer dimer formation was predicted us<strong>in</strong>g thePrimerSelect module <strong>of</strong> DNASTAR (Madison, WI). Specificity<strong>of</strong> real-time amplifications was tested on a Roche DiagnosticsLightCycler 1, us<strong>in</strong>g DNA extracted from a range <strong>of</strong>related <strong>Pythium</strong> species, previously isolated from g<strong>in</strong>seng<strong>soils</strong> (Table 1).In order to confirm target amplification from soil, thealignment <strong>of</strong> <strong>Pythium</strong> species used for taxon specific primerdesign was also used to identify restriction sites whichcould dist<strong>in</strong>guish between the amplified DNA <strong>of</strong> our targetspecies and that <strong>of</strong> other <strong>Pythium</strong> species from naturally<strong>in</strong>fested <strong>soils</strong>. Restriction digests <strong>of</strong> the ITS region withenzymes FspI and BccI were predicted to result <strong>in</strong> restrictiondigestion patterns characteristic <strong>of</strong> P. irregulare Groups I andII (sensu Matsumoto et al., 2000) andP. ultimum, respectively.To test for the presence <strong>of</strong> these restriction sites, digests <strong>of</strong>PCR products generated from 17 <strong>Pythium</strong> isolates byconventional PCR (Table 1) were carried out by comb<strong>in</strong><strong>in</strong>g10 mL <strong>of</strong>PCRproductwith5U<strong>of</strong>eitherFspIorBccI(NewEngland Biolabs, Picker<strong>in</strong>g, Ontario), 2 mL <strong>of</strong> 10 reactionbuffer and 7 mL H 2 O. Reactions were <strong>in</strong>cubated at 37 8C for3 h, separated electrophoretically on 2% agarose <strong>in</strong> TAEbuffer and analyzed with the Gel Doc EQ System (Bio-Rad,Hercules, CA).2.3. Collection and preparation <strong>of</strong> soilArtificially <strong>in</strong>fested soil was prepared us<strong>in</strong>g Fox loamy sand(Brunosolic Gray Brown Luvisol; Typic Hapludalf; 0.9% OM)from the Agriculture and Agri-food Canada research farm atDelhi, Ontario (42847 0 N, 80838 0 W). Soil was steam pasteurizedat 74 8C for 30 m<strong>in</strong> us<strong>in</strong>g a L<strong>in</strong>dig soil treatment system (L<strong>in</strong>digManufactur<strong>in</strong>g, St. Paul, MN), air-dried, sieved through a 4 mmmesh, mixed and stored at room temperature until used. Soildilution (1 part soil <strong>in</strong> 4 parts 0.25% water agar) procedureswere carried out us<strong>in</strong>g P 5 ARP semi-selective media (Jeffers andMart<strong>in</strong>, 1986; Reeleder et al., 2006b) to confirm that pasteurizationhad elim<strong>in</strong>ated viable propagules. P. irregulare (PID008)and P. ultimum (PID095) were grown for 2 weeks <strong>in</strong> clarified V8broth. Twenty mycelial mats were then washed with sterilewater and vacuum filtration, macerated us<strong>in</strong>g autoclavedm<strong>in</strong>i-blenders and re-suspended <strong>in</strong> approx. 240 mL sterilewater. Macerates were mixed <strong>in</strong>to pasteurized soil atapproximately 40 mL/kg soil <strong>in</strong> a large plastic bag and mixedby vigorously shak<strong>in</strong>g, then allow<strong>in</strong>g the mixed soil to air dry.The result<strong>in</strong>g <strong>in</strong>oculum was then further mixed withpasteurized field soil <strong>in</strong> different proportions. The result<strong>in</strong>gsoil dilutions were 100, 50, 10 and 1%. Each dilution was thendivided <strong>in</strong>to six sub-samples, air dried <strong>in</strong> the greenhouse andstored at 4 8C until plat<strong>in</strong>g and DNA extraction.Samples <strong>of</strong> naturally <strong>in</strong>fested <strong>soils</strong> (rang<strong>in</strong>g from sands tosandy loams) were collected at seven sites <strong>in</strong> south-westernOntario. At each site, one 441 m 2 plot was constructed anddivided <strong>in</strong>to 49.9 m 2 quadrats. Approx. 2 L <strong>of</strong> topsoil wasremoved from each quadrat. Sampl<strong>in</strong>g locations wererecorded by GPS. Soil samples from with<strong>in</strong> each site werepooled, passively air dried at room temperature, mixed with acommercial cement mixer, sieved, and mixed aga<strong>in</strong>. Allequipment, <strong>in</strong>clud<strong>in</strong>g mixer and sieve were sterilized withhousehold bleach (diluted with water to 0.5% sodium hypochlorite)between <strong>soils</strong>. Soils were placed <strong>in</strong> plastic bags andstored at 4 8C until used. DNA was extracted and purified from5 g subsamples <strong>of</strong> each artificially and naturally <strong>in</strong>fested soilas described <strong>in</strong> Kernaghan et al. (2007).2.4. qPCRqPCR was performed on extracts <strong>of</strong> samples <strong>of</strong> the artificially<strong>in</strong>fested soil us<strong>in</strong>g SYBR green chemistry on a RocheDiagnostics Light Cycler TM I. Twenty microlitres reactionmixtures were prepared <strong>in</strong> glass capillaries (Roche Diagnostics)and <strong>in</strong>cluded 2 mL soil DNA extract, 9.8 mL Quanti-Tect SYBR Green PCR Master Mix (Qiagen), 0.1 U HotStarTaqDNA Polymerase (Qiagen) and 0.6 mM each <strong>of</strong> primers PiFand PiR or PuF and PuR. Reactions us<strong>in</strong>g PuF/PuR received1mM MgCl 2 <strong>in</strong> addition to the 2.5 mM present <strong>in</strong> theQuantiTect Master Mix.For each primer pair, PCR reactions were carried out onDNA extractions from each <strong>of</strong> the six subsamples taken fromeach soil dilution, as well as on a dilution series <strong>of</strong> referenceDNA (Quantified DNA from pure culture) and the negative(yeast) control extract. All reactions received a 15 m<strong>in</strong> pretreatmentat 95 8C, then 60 cycles <strong>of</strong> 94 8C for 15 s, 64 8C for 15 s,72 8C for 25 s for the PiF/PiR primer set and 94 8C for 15 s, 54 8Cfor 30 s, 72 8C for 35 s for the PuF/PuR primer set. For all