Solutions
Solutions
Solutions
You also want an ePaper? Increase the reach of your titles
YUMPU automatically turns print PDFs into web optimized ePapers that Google loves.
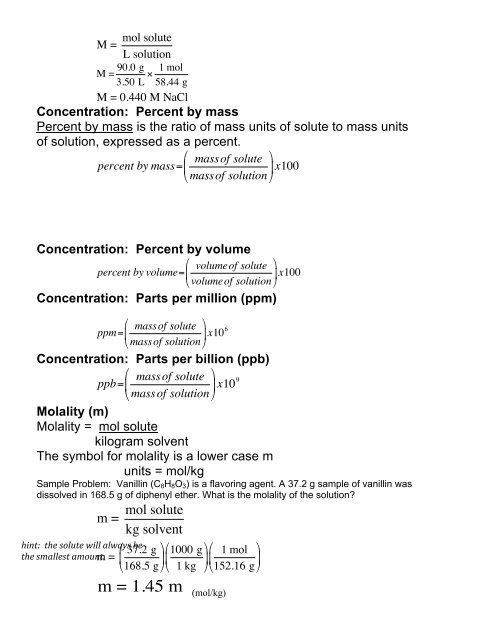
M =mol soluteL solutionM = 90.0 g3.50 L ! 1 mol58.44 gM = 0.440 M NaClConcentration: Percent by massPercent by mass is the ratio of mass units of solute to mass unitsof solution, expressed as a percent.! massof solute $percent by mass= #&x100" massof solution %Concentration: Percent by volume! volumeof solute $percent by volume= #&x100" volumeof solution %Concentration: Parts per million (ppm)! massof solute $ppm= #&x10 6" massof solution %Concentration: Parts per billion (ppb)! massof solute $ppb= #&x10 9" massof solution %Molality (m)Molality = mol solutekilogram solventThe symbol for molality is a lower case munits = mol/kgSample Problem: Vanillin (C 8 H 8 O 3 ) is a flavoring agent. A 37.2 g sample of vanillin wasdissolved in 168.5 g of diphenyl ether. What is the molality of the solution?mol solutem =kg solventhint: the solute will always be the smallest amount.m =! 37.2 g $ !# & 1000 g $ ! 1 mol $# &#&" 168.5 g %"1 kg %"152.16 g %m = 1.45 m(mol/kg)