An 11-year-old Girl Who Has Left Leg Pain
An 11-year-old Girl Who Has Left Leg Pain
An 11-year-old Girl Who Has Left Leg Pain
Create successful ePaper yourself
Turn your PDF publications into a flip-book with our unique Google optimized e-Paper software.
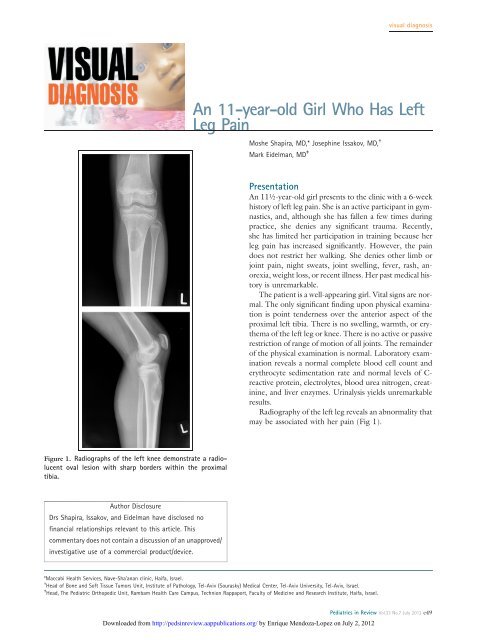
visual diagnosis<strong>An</strong> <strong>11</strong>-<strong>year</strong>-<strong>old</strong> <strong>Girl</strong> <strong>Who</strong> <strong>Has</strong> <strong>Left</strong><strong>Leg</strong> <strong>Pain</strong>Moshe Shapira, MD,* Josephine Issakov, MD, †Mark Eidelman, MD ‡Presentation<strong>An</strong> <strong>11</strong>½-<strong>year</strong>-<strong>old</strong> girl presents to the clinic with a 6-weekhistory of left leg pain. She is an active participant in gymnastics,and, although she has fallen a few times duringpractice, she denies any significant trauma. Recently,she has limited her participation in training because herleg pain has increased significantly. However, the paindoes not restrict her walking. She denies other limb orjoint pain, night sweats, joint swelling, fever, rash, anorexia,weight loss, or recent illness. Her past medical historyis unremarkable.The patient is a well-appearing girl. Vital signs are normal.The only significant finding upon physical examinationis point tenderness over the anterior aspect of theproximal left tibia. There is no swelling, warmth, or erythemaof the left leg or knee. There is no active or passiverestriction of range of motion of all joints. The remainderof the physical examination is normal. Laboratory examinationreveals a normal complete blood cell count anderythrocyte sedimentation rate and normal levels of C-reactive protein, electrolytes, blood urea nitrogen, creatinine,and liver enzymes. Urinalysis yields unremarkableresults.Radiography of the left leg reveals an abnormality thatmay be associated with her pain (Fig 1).Figure 1. Radiographs of the left knee demonstrate a radiolucentoval lesion with sharp borders within the proximaltibia.Author DisclosureDrs Shapira, Issakov, and Eidelman have disclosed nofinancial relationships relevant to this article. Thiscommentary does not contain a discussion of an unapproved/investigative use of a commercial product/device.*Maccabi Health Services, Nave-Sha’anan clinic, Haifa, Israel.† Head of Bone and Soft Tissue Tumors Unit, Institute of Pathology, Tel-Aviv (Sourasky) Medical Center, Tel-Aviv University, Tel-Aviv, Israel.‡ Head, The Pediatric Orthopedic Unit, Rambam Health Care Campus, Technion Rappaport, Faculty of Medicine and Research Institute, Haifa, Israel.Downloaded from http://pedsinreview.aappublications.org/ by Enrique Mendoza-Lopez on July 2, 2012Pediatrics in Review Vol.33 No.7 July 2012 e49
visual diagnosisDiagnosis: Bone Cyst of Proximal TibiaRadiography reveals a radiolucent oval lesion; it is mostlikely a unicameral bone cyst (UBC). There is no evidenceof a fracture. The patient is referred to a pediatricorthopedist for further evaluation.DiscussionDescribed by Virchow in 1876, a UBC (also known asa simple bone cyst, solitary bone cyst, or juvenile bonecyst) is a benign fluid-filled cavity found primarily in themetaphyseal region of the long bones, especially in skeletallyimmature children. This type of cyst tends to expandand weaken the bone, thereby predisposing it to a pathologicfracture. (1)(2) UBCs appear more frequently inmales than in females (2.5:1), and occur primarily in patientsunder age 20 <strong>year</strong>s (85%), with a peak incidenceat age 10 <strong>year</strong>s. (1)(3) This lesion accounts for 3% ofbiopsied bone tumors. Typical cyst locations include thehumerus, femur, tibia, and fibula. (2)(3) Less commonlocations include the calcaneus (2%–3% of cases), (2)distal radius, (4) and spine. (5)Etiology and PathogenesisThe cause of formation of a UBC is unclear, but theoriesinclude venous obstruction, development of an intraosseoussynovial cyst, dysplastic bone formation in responseto trauma, and localized failure of ossification during a periodof rapid growth. (1)(2)(3) The fluid within the cystcontains oxygen-free radical scavengers, prostaglandins,interleukin-1, and proteolytic enzymes that cause bone resorption,thereby contributing to the formation andgrowth of the cyst. (1) Microscopic examination of thecyst walls reveals primarily fibrous tissue with occasionalgiant cells. (1)(2)Clinical and Radiologic FeaturesThe UBC usually is asymptomatic and is detected oftenby incidental radiographic imaging of a limb for reasonsnot related to the cyst, such as minor trauma or limp.<strong>An</strong>y associated pain may be due to a pathologic fracturethrough the cyst or to microfractures not yet detectedby radiograph.On radiograph, the bone cyst appears as a round orovoid lucent lesion with an overlying thin, expanded corticalbone. Occasionally, when there is a fracture, a fragmentof the cyst wall appears within the fluid cavity, evident asa “fallen leaf” radiographic sign. (1)(2) Computed tomographyand magnetic resonance imaging will show the lesionto be fluid filled with a thin enhancing wall, but are oftennot necessary for diagnosis due to the characteristic appearanceof UBC on plain radiographs. Sometimes a few septationsare present. (2)Seventy-five percent of patients who have UBC presentwith a pathologic fracture. (6) Although the proximalmetaphysis is the classic location of most UBCs, therehave been reports of epiphyseal involvement. Ovadiaand associates (7) reported eight children with a medianage at diagnosis of 8.4 <strong>year</strong>s having bone cysts crossingthe growth plate. All children had more than two pathologicfractures; seven patients had growth disturbancewithout functional impairment.A UBC is defined as active if the cyst has increased inlength or width by 25% in serial radiologic studies, if thepatient has functional pain, if pathologic fractures appearwithout resolution of the cyst, or if the cyst is associatedwith cortical thinning (impending fracture). (6) In addition,a cyst is considered active if it has close proximity tothe epiphyseal plate, and is considered latent if it is >0.5 cmdistal from the physis. (6) Wilkins states that it is radiographicallydifficult to assess the current stage (growthversus involution) of the cyst at the time of diagnosis,a status that has implications regarding treatment andpossible complications. (1)Differential DiagnosisThe differential diagnosis for UBC includes an aneurysmalbone cyst, which is a benign osteolytic boneneoplasm characterized by expansive hemorrhagic,lobulated tissue arising in bone, and fibrous dysplasia,which is a benign disorder characterized by presence ofintramedullary fibro-osseous tissue that arises in the diaphysis.(6)TreatmentConservative management with close outpatient followupis the treatment of choice for the asymptomatic,physically active child who has a small cyst with thickenedwalls (asymptomatic UBC), because chances aregood that the cyst is in its involutional stage and willsoon disappear. (1)(3) However, if there is increasedrisk for a pathologic fracture through the thin cortex,treatment should occur to prevent fracture and possiblecomplications, such as residual skeletal deformity. (1)(3)Sites of particular concern are lesions in the proximal femurand the dominant throwing arm of athletes, due tothehighstressesattheselocationsandanelevatedriskof pathologic fracture. If presentation involves a pathologicfracture, fracture treatment occurs first, reservinge50 Pediatrics in Review Vol.33 No.7 July 2012Downloaded from http://pedsinreview.aappublications.org/ by Enrique Mendoza-Lopez on July 2, 2012
visual diagnosisFigure 2. Axial and sagittal computed tomographic sectionsof left leg confirming UBC morphology.treatment of the cyst for those that persist after fracturehealing.Treatment options are (1) percutaneous aspiration andlocal corticosteroid or autogenous bone marrow injectionFigure 4. <strong>Left</strong> leg radiograph confirming surgical filling of UBC.Figure 3. Histology of UBC. The cyst wall is composed offibrous membrane (black arrow) bordered by bony trabeculae.Fibrin-like material and a few osteoclast-like giant cells arepresent within the cyst (red arrow).and (2) open curettage and bone grafting (autografting,allografting, or use of an artificial bone substitute suchas calcium sulfate). A new modified procedure consistingof minimally invasive curettage, ethanol cauterization,disruption of the cystic boundary, insertion ofa synthetic calcium sulfate bone graft substitute, andplacement of a cannulated screw to provide drainagehas been described recently. This operative procedureshowed the highest radiographic healing rate in comparisonwith the other methods discussed. (3) Donaldson,in a review of treatment options, states that corticosteroidinjection is the only evidence-based treatment for UBCs,but treatment strategies that include opening the medullarycanal, disrupting the cyst wall, and filling the lytic defectwith a bone substitute may be preferable; however,evidence is still lacking. (8)Successful healing of a UBC after surgical interventionas judged by radiograph ranges from 29% to 100%. Complicationsinclude physeal injury and cyst recurrence.Children who received instrumentation as part of theirtreatment eventually require implant removal. (3) Longtermclinical and radiographic follow-up is recommended,because recurrence of UBC has been reported in 18% to88% of patients at 6 months to 7 <strong>year</strong>s after the initialDownloaded from http://pedsinreview.aappublications.org/ by Enrique Mendoza-Lopez on July 2, 2012Pediatrics in Review Vol.33 No.7 July 2012 e51
visual diagnosistreatment. (3) Subsequent fracture after surgery is anothercomplication and can affect a child’s quality of life adversely;a child may avoid physical activities for fear ofcausing a fracture. (1)Patient CourseBased on the clinical examination and the radiographic picture,the diagnosis of UBC was established and the patientwas asked to restrict physical activities. She returned 3months later complaining of pain, even without physicaleffort. Computed tomography demonstrated a hypodenselesion with a sclerotic border measuring 22 52 21 mmlocated in the metaphysis (Fig 2). A repeat radiograph ofthe leg showed a slight increase in the cyst’s size withoutevidence of an obvious pathologic fracture. The patientunderwent cyst curettage with bone grafting. Pathologicexamination confirmed the diagnosis of UBC, showingthat the wall of the cyst was composed of fibrous membranesbordered by the bony trabeculae. Fibrin-like materialand a few osteoclast-like giant cells were seen focally as well(Fig 3). The postoperative period was uneventful. Two<strong>year</strong>s later, the patient no longer has leg pain and has returnedto full physical activities. A follow-up radiographconfirmed filling of the cavity without complications(Fig 4).Summary• Unicameral bone cysts (UBCs) in children usually areasymptomatic. Most UBCs are discovered whena radiograph is performed on a child who has hadaccidental trauma to a limb.• Symptomatic cysts typically present with pain, oftenthe result of pathologic fracture through a large cystor occult stress fracture within the thinned cortexaround the cyst.• Simple radiography is the best method for detectingsuch cysts, which typically are located within the longbone (femur, tibia, fibula, humerus), but can appearelsewhere.• Cysts typically appear in the proximal metaphysis, butsome involve the epiphysis and growth plate, therebyaffecting bone growth.• If clinically necessary to confirm the diagnosis,computed tomography or magnetic resonanceimaging can delineate the cyst better or demonstratean occult fracture.• For the asymptomatic UBC, close follow-up is therecommended course of action. However, surgicalintervention by corticosteroid or autogenous bonemarrow injection or open curettage with bonegrafting is recommended if the cyst is symptomatic,carries an increased risk for pathologic fracture(weight-bearing bone or dominant arm of a throwingathlete), or shows signs of an impending pathologicfracture.• Clinical and radiographic follow-up is recommendedafter surgical intervention, because UBC recurrenceafter initial surgery is reported to occur in18% to 88%of patients.References1. Wilkins RM. Unicameral bone cysts. J Am Acad Orthop Surg.2000;8(4):217–2242. Schwartz D. <strong>An</strong> <strong>11</strong>-<strong>year</strong>-<strong>old</strong> boy with ankle trauma: unicameralbone cyst of the calcaneus. Pediatr <strong>An</strong>n. 2009;38(3):132–134,1343. Hou HY, Wu K, Wang CT, Chang SM, Lin WH, Yang RS.Treatment of unicameral bone cyst: a comparative study of selectedtechniques. J Bone Joint Surg Am. 2010;92(4):855–8624. Cohen N, Keret D, Ezra E, Lokiec F. Unusual simple bonecyst of the distal radius in a toddler. Isr Med Assoc J. 2002;4(2):137–1395. Ha KY, Kim YH. Simple bone cyst with pathologic lumbarpedicle fracture: a case report. Spine. 2003;28(7):E129–E1316. Ortiz EJ, Isler MH, Navia JE, Canosa R. Pathologic fractures inchildren. Clin Orthop Relat Res. 2005;432(432):<strong>11</strong>6–1267. Ovadia D, Ezra E, Segev E, et al. Epiphyseal involvement ofsimple bone cysts. J Pediatr Orthop. 2003;23(2):222–2298. Donaldson S, Wright JG. Recent developments in treatment forsimple bone cysts. Curr Opin Pediatr. 20<strong>11</strong>;23(1):73–77Suggested ReadingGereige R, Kumar M. Bone lesions: benign and malignant. PediatrRev. 2010;31(9):355–363e52 Pediatrics in Review Vol.33 No.7 July 2012Downloaded from http://pedsinreview.aappublications.org/ by Enrique Mendoza-Lopez on July 2, 2012
Articlegastrointestinal disordersConjugated Hyperbilirubinemia in ChildrenDavid Brumbaugh, MD,*Cara Mack, MD*Author DisclosureDrs Brumbaugh andMack have disclosedno financialrelationships relevantto this article. Thiscommentary does notcontain a discussion ofan unapproved/investigative use ofa commercial product/device.Education Gaps1. Awareness of telltale signs and performance of appropriate diagnostic testing canhelp clinicians identify neonatal cholestasis in time to ameliorate its potentiallycatastrophic outcomes.2. The success of the Kasai procedure to restore bile flow is directly related to patient age:at less than 60 days after birth, two-thirds of patients benefit from the procedure;however, at 90 days after birth, chances for bile drainage diminish markedly.Objectives After completing this article, readers should be able to:1. Understand the metabolism of bilirubin, the differences between conjugated andunconjugated bilirubin, and the relationship of conjugated hyperbilirubinemia tocholestasis.2. Delineate the causes of cholestasis in the newborn and know how to evaluate thecholestatic neonate.3. Manage the infant who has prolonged cholestasis.4. Understand the causes of conjugated hyperbilirubinemia in the <strong>old</strong>er child andadolescent and know how to assess children who have conjugated hyperbilirubinemia.IntroductionCentral to human digestive health are both the production of bile by hepatocytes and cholangiocytesin the liver and the excretion of bile through the biliary tree. By volume, conjugatedbilirubin is a relatively small component of bile, the yellowish-green liquid that alsocontains cholesterol, phospholipids, organic anions, metabolized drugs, xenobiotics, and bileacids. In most cases, the elevation of serum-conjugated bilirubin is a biochemical manifestationof cholestasis, which is the pathologic reduction in bile formation or flow.Complex mechanisms exist for the transport of bile componentsfrom serum into hepatocytes across the basolateralAbbreviationscell surface, for the trafficking of bile components throughthe hepatocyte, and finally for movement of these bile componentsacross the apical cell surface into the bile canaliculus,AIH: autoimmune hepatitisALT: alanine aminotransferasewhich is the smallest branch of the biliary tree. From the bileAST: aspartate aminotransferasecanaliculus, bile then flows into the extrahepatic biliary tree,A1AT: alpha-1 antitrypsinincluding the common bile duct, before entering the duodenumat the ampulla of Vater (Fig 1). Isolated gene defectsBA: biliary atresiaBRIC: benign recurrent intrahepatic cholestasisin proteins responsible for trafficking bile components canBSEP: bile salt excretory proteinlead to cholestatic diseases.CDC: choledochal cystERCP: endoscopic retrograde cholangiopancreatographyGGT: gamma glutamyltransferaseMCT: medium chain triglyceridesMRCP: magnetic resonance cholangiopancreatographyPFIC: progressive familial intrahepatic cholestasisPN: parenteral nutritionPSC: primary sclerosing cholangitisDiagnosisUnconjugated bilirubin is the product of heme breakdown,and this molecule, poorly soluble in water, is carried in thecirculation principally as a water-soluble complex joined withalbumin. Unconjugated bilirubin is then taken up into hepatocytes,where a glucuronic acid moiety is added, renderingthe conjugated bilirubin water soluble. Conjugated*Digestive Health Institute, Children’s Hospital of Colorado, University of Colorado <strong>An</strong>schutz Medical Campus, Denver, CO.Pediatrics in Review Vol.33 No.7 July 2012 291
gastrointestinal disordersconjugated hyperbilirubinemiaFigure 1. Biliary drainage with magnification of portal triad. (Courtesy of Robert E. Kramer, MD.)hyperbilirubinemia is defined biochemically as a conjugatedbilirubin level of ‡2 mg/dL and >20% of the totalbilirubin. There are two commonly used laboratory techniquesto estimate the level of conjugated bilirubin. Thefirst uses spectrophotometry to measure directly conjugatedbilirubin. The laboratory may also estimate a “direct”bilirubin, which reflects not just conjugated bilirubin butalso delta-bilirubin, which is the complex of conjugatedbilirubin and albumin. Hence, the “direct” bilirubin willtend to overestimate the true level of conjugated hyperbilirubinemia,and in neonates is less specific for the presenceof underlying hepatobiliary disease. (1)With the exception of Rotor and Dubin-Johnson syndromes,discussed later in this article, the elevation ofserum-conjugated bilirubin reflects cholestasis. The presenceof cholestasis may be a manifestation of generalizedhepatocellular injury, may reflect obstruction to bile flow atany level of the biliary tree, or may be caused by a specificproblem with bile transport into the canaliculus. Systemicdisease leading to hypoxia or poor circulatory flow also canimpair bile formation and lead to cholestasis.Recognition of the Cholestatic NewbornOwing to immaturity of the excretory capability of theliver, the newborn is particularly prone to the developmentof cholestasis. The challenge for the primary care clinicianis prompt recognition of the cholestatic infant. Observationof stool color is a necessary component of the initialassessment, because acholic stools represent significantcholestasis. Furthermore, hepatomegaly, with or withoutsplenomegaly, often is identified in the setting ofcholestasis.292 Pediatrics in Review Vol.33 No.7 July 2012
gastrointestinal disordersconjugated hyperbilirubinemiaforms of BA. The embryonic form of BA, which is associatedwith other congenital anomalies such as heterotaxysyndrome and polysplenia, accounts for w15% to 20%of BA.The acquired form of BA is far more common (w85%);the etiology of this disease is unclear. The pathophysiologyof acquired BA is that of a brisk inflammatory response involvingboth the intra- and extrahepatic bile ducts. Theducts are destroyed gradually and replaced with fibrous scartissue. The lumen of the bile duct is eventually obliterated,and normal bile flow is impaired, leading to cholestasis.Infants who have acquired BA typically are asymptomaticat birth and develop jaundice in the first weeks afterbirth. Typically, they feed well and thrive. As the bile flowdiminishes, the stool color loses its normal pigmentationand becomes acholic, or clay-colored. The finding ofacholic stools in the setting of a jaundiced newborn shouldprompt expedient evaluation for BA. Light-colored stoolsmay not be appreciated by the inexperienced parent,and the stool should be examined by the primary care providerto assess pigmentation.In Taiwan, which has one of the highest incidences ofBA in the world, a universal screening program providesparents with a stool color card on discharge from the newbornnursery (Fig 2). At 1 month of age, the parents returnthe stool card to their provider after marking the picture ofthe stool color that most closely resembles the infant’s stool.The universal screening program has led to improvement ofearly detection of infants who have BA, which has resultedin dramatic improvement in surgical outcomes. (4)Evaluation for BA includes abdominal ultrasonographyto rule out other anatomic abnormalities of the commonbile duct, such as choledochal cyst (CDC), and to identifyanomalies associated with the embryonic form of BA.A liver biopsy often is performed, and histopathologicfindings consistent with BA include bile ductular proliferation,portal tract inflammation and fibrosis, and bile plugswithin the lumen of bile ducts. The g<strong>old</strong> standard in confirmingthe diagnosis of BA is the intraoperative cholangiogram,which shows obstruction of flow within a segmentor the entirety of the extrahepatic bile duct.A Kasai portoenterostomy is then performed in an attemptto reestablish bile flow. This operation entails excisionof the fibrous bile duct followed by anastomosis ofa loop of jejunum to the base of the liver in a Roux-en-Yfashion. The success of this surgery, which is the restorationof bile flow to intestine, is directly related to the age of thepatient. Early Kasai procedure, defined as
gastrointestinal disordersconjugated hyperbilirubinemiapoor weight gain, ascites, acholic stools, and vomiting.Ultrasonography typically reveals ascites and fluid aroundthe gallbladder. Bile-stained ascitic fluid is a hallmarkfinding. The treatment of both of these conditions involvessurgical intervention.Stagnant flow of bile leading to cholestasis is seen oftenin the setting of intestinal disease and parenteral nutrition(PN) in the neonate. Precipitation of cholesterol and calciumsaltswithinbilecanresultintheformationofsludge.Bile sludge can be detected by ultrasonography. Whensludge builds up and leads to biliary obstruction and the developmentof cholestasis, the patient is said to have inspissatedbile syndrome. Inspissated bile can be managedconservatively with ursodeoxycholic acid, a bile salt that actsas a choleretic agent to promote bile flow. Because inspissatedbile syndrome can mimic biliary atresia, the diagnosissometimes is made at the time of intrahepatic cholangiogram,and saline flushes of the biliary tree by the surgeon canprovide the definitive therapy. The use of third-generationcephalosporin antibiotics, in particular ceftriaxone, has beenassociated with the formation of bile sludge in newborns.InfectionsNeonatal cytomegalovirus infection,vertically acquired from themother, is the most common congenitalinfectious cause of neonatalcholestasis. <strong>An</strong>y of the conditionsformerly identified as the “TORCH”family of infections (toxoplasmosis,rubella, cytomegalovirus, herpesvirus,syphilis) can lead to a similarpattern of cholestasis and growthrestriction. Acquired infections afterbirth can lead to cholestasis, inparticular Gram-negative infectionsassociated with urinary tract infectionsand sepsis, because hepatic bileflow is very sensitive to circulatingendotoxins.The characteristic finding on liver histology is paucity ofbile ducts. The clinical course of liver disease in infantswhohavealagillesyndromeishighlyvariable,withsomechildren experiencing a gradual improvement in cholestasisin childhood, whereas others progress to cirrhosis, requiringliver transplantation in childhood. Infants born withTrisomy 21 also are at increased risk for development ofa paucity of intrahepatic bile ducts; however, this situationusually is very mild, with resolution of cholestasis in infancy.Cystic fibrosis is another genetic disorder that canpresent with neonatal cholestasis and often is associatedwith meconium plug syndrome. Early diagnosis is aidedby the availability in all 50 states of newborn screeningfor cystic fibrosis by measurement of immunoreactivetrypsinogen levels.Along the apical surface of the hepatocyte, there arespecific transporter proteins that are responsible for trafficof bile components into the bile canaliculus (Fig 3). (5)Defects in these proteins are associated with cholestaticdisease. For instance, a mutation in the gene coding forbile salt excretory protein (BSEP) interferes with bile salttrafficking into the canaliculus, leading to reduced bileflow and the toxic accumulation of hydrophobic bile acidsGenetic DisordersAlagille syndrome is an autosomaldominant mutation of the Jagged 1gene on chromosome 20. There isvariable penetrance of this mutation,which can lead to abnormalities ofthe liver (cholestasis), heart (peripheralpulmonary stenosis), skeletal system(butterfly vertebrae), kidneys,and eyes (posterior embryotoxin).Figure 3. Canalicular membrane surface proteins, their substrates, and knownassociations with pediatric disease. (For a recent review, see Wagner M, Zollner G,Trauner M. New molecular insights into the mechanisms of cholestasis. J Hepatol.2009;51:565–580.)Pediatrics in Review Vol.33 No.7 July 2012 295
gastrointestinal disordersconjugated hyperbilirubinemiawithin hepatocytes. This mutation produces the clinicalphenotype of cholestasis and pruritis in the first <strong>year</strong> afterbirth, a condition known as PFIC2.A defect in the gene coding for FIC1, another canalicularsurface protein, produces the clinical phenotypePFIC1, which, in addition to cholestasis, can present withdiarrhea and growth failure. Pruritis, a dominant clinicalfeature of both PFIC1 and PFIC2, typically is not problematicuntil after 6 months of age.PFIC3 is a syndrome caused by a defect in the genecoding for the transporter MDR3, which is responsiblefor phosphatidylcholine secretion into the bile canaliculus.The onset of cholestasis is variable in PFIC3 but typicallyoccurs later than in PFIC1 and PFIC2. In contrast toPFIC1 and PFIC2, which are featured by a GGT levelin the low or normal range, the GGT in PFIC3 is elevated.Metabolic DisordersA range of metabolic diseases can present initially as cholestasisin the newborn period and are associated withgene mutations in most cases (thus, these diseases couldalso fall under the category of genetic disorders). Persistentjaundice in the newborn period is one of the earliestpotential clinical manifestations of alpha-1 antitrypsin(A1AT) deficiency, a defect in the “ATZ” molecule thatresults in abnormal accumulation of A1AT in the endoplasmicreticulum of hepatocytes. The abnormal retentionof A1AT within the hepatocyte leads to abnormalbile formation and secretion.Inborn errors of metabolism, which include disordersof fatty acid oxidation, tyrosinemia, and galactosemia,among others, can present in the neonatal period with aspectrum of liver disease that includes cholestasis. Finally,bile acid synthesis defects often present with neonatal cholestasis.As the production of bile acids from cholesteroland their subsequent export into the canaliculus are therate-limiting steps in bile flow, defects in a number of enzymaticsteps within this pathway result in abnormal bileacid synthesis and cholestasis. Bile acid synthesis defectsgenerally can be treated effectively by oral bile acidsupplementation.EndocrinopathiesCongenital endocrinopathies must be considered in thedifferential diagnosis of neonatal conjugated hyperbilirubinemia.Neonatal cholestasis is a well-recognizedmanifestation of congenital hypothyroidism. Congenitalpanhypopituitarism is manifested by deficiencies in cortisol,growth hormone, and thyroid hormone. These hormoneshave been shown to promote bile formation or secretionand chronic deficiencies lead to cholestasis. Other clinicalfindings associated with panhypopituitarism include opticnerve hypoplasia, septo-optic dysplasia, and, in male patients,microphallus. In contrast to most of the cholestaticdiseases, which lead to an elevation in serum GGT concentrations,the GGT level in hypopituitarism typically is normal.Hypoglycemia can complicate prolonged fasts in theseinfants.Drug HepatotoxicityDependent on the maturity of the neonate, there is variabilityin the activity of members of the drug-metabolizingcytochrome P450 family in the newborn period. Thus, thenewborn infant may be particularly susceptible to druginducedhepatotoxicity, which can take a predominantlycholestatic form. The most common drug-induced liverinjury is caused by PN, used in the newborn period for avariety of indications. The liver injury caused by PN ismultifactorial, but in particular the phytosterol present insoy-based lipid formulations is a known antagonist of thenuclear receptor FXR, which is a regulator of the BSEP,an essential protein involved in bile acid transport. (6)Initial experience using fish oil–based sources of intravenouslipids has been promising, but larger clinical trialsin neonates have not yet been performed. There are casereports of neonatal cholestasis associated with maternal useof prescribed medications (carbamazepine) and illicitdrugs (methamphetamine). Postnatal infant exposureto antimicrobial agents, particularly ceftriaxone, fluconazole,and micafungin, has been associated with the developmentof cholestasis.Management of the Infant <strong>Who</strong> <strong>Has</strong>Prolonged CholestasisFailure to thrive is found commonly in infants who havechronic cholestatic conditions. The cause of poor weightgain is multifactorial. Reduced bile flow to the intestine resultsin poor solubilization of dietary fats in mixed micelles,leading to fat malabsorption and steatorrhea. Mediumchaintriglycerides (MCT) do not require bile for intestinalabsorptionandthusarepreferredininfantswhohavecholestasis.Several commercially available formulas have highlevels of MCT as their fat source, and there are supplementscontaining exclusively MCT that can be used for delivery ofadditional calories.Infants who have chronic liver disease may have an increasedbaseline caloric need coupled with demands for additionalcalories for catch-up growth. Unfortunately, manyof these infants are anorexic, justifying the use of nasogastricfeeds for caloric delivery. Fat-soluble vitamin deficienciesare pervasive in infants who have chronic cholestasis andshould be managed aggressively with frequent monitoring296 Pediatrics in Review Vol.33 No.7 July 2012
gastrointestinal disordersconjugated hyperbilirubinemiaof serum vitamin levels and use of oral fat-soluble vitaminsupplements.Ursodeoxycholic acid is a hydrophilic bile acid that isuseful in managing many cholestatic conditions. This bileacid has two purported benefits. First, it can stimulate bileflow and reduce cholestasis; second, it may displace moretoxicbile acids from the hepatocyte, thus potentially lesseningthe hepatocyte injury associated with cholestasis. Forsevere pruritis seen in cholestasis, which is caused by thedeposition of bile acids in the skin, oral antihistamines provideno benefit. Ursodeoxycholic acid can be helpful, andthe oral antibiotic rifampin often is added for refractorypruritis. The action of this agent in reducing itching is stillincompletely understood; but rifampin has been shown toprovide dramatic relief for affected infants.Approach to the Child and Adolescent <strong>Who</strong><strong>Has</strong> Conjugated HyperbilirubinemiaOutside of infancy, conjugated hyperbilirubinemia is amuch less common laboratory finding. Depending onthe cause of the hyperbilirubinemia, clinical manifestationswill vary and can include scleral icterus, jaundice, fatigue,pruritis, abdominal pain, and nausea. Chronicity of diseasecan be assessed by the history, keeping in mind that in thesetting of hepatobiliary disease, a nonspecific symptom,such as fatigue, may be present months before the developmentof more objective symptoms of cholestasis, such asjaundice and pruritis.The physical examination should include assessment ofliver size and texture. A firm, nodular liver suggests chronichepatobiliary disease and the development of cirrhosis.Physical stigmata of portal hypertension and cirrhosis includesplenomegaly, ascites, palmar erythema, caput medusae,and spider angioma. Normal metabolism of thesteroid intermediate androstenedione to testosterone typicallyoccurs in the liver. In end-stage liver disease, moreandrostenedione is eventually converted to estradiol, leadingto the development of gynecomastia in male patients.In female adolescents, secondary amenorrhea may resultfrom chronic liver disease.Initial laboratory assessment will include the measurementof serum aminotransferases (AST, ALT), GGT,and bilirubin (including conjugated, or direct bilirubin),as well as performing tests of liver synthetic function, includingprothrombin time and serum albumin level. Patientswho have poor liver synthetic function, manifestedas an elevated prothrombin time or low serum albuminlevel, should be referred urgently to a center with expertisein pediatric hepatology.If the physical examination and initial laboratory findingsdo not support chronic liver disease, but there is anelevated direct bilirubin fraction, consider a defect in thecanalicular transport of bilirubin. (7) Dubin-Johnson syndromeis a defect in the anion transporter gene ABCC2,inherited in an autosomal recessive fashion, which leadsto elevation both of unconjugated and conjugated bilirubinlevels. Rotor syndrome has a similar presentation tothat of Dubin-Johnson syndrome, but the underlying geneticdefect is unknown. These syndromes involve problemsin the storage/excretion of conjugated bilirubin andpresent with normal aminotransferase levels and the absenceof pruritis. The principal clinical objective is to distinguishthese benign conditions from the serious hepatobiliary diseasesdiscussed later in this article.The initial evaluation of a child or adolescent who hasconjugated hyperbilirubinemia should include abdominalultrasonography to assess for obstruction of the biliary tree.Typical symptoms reported with biliary obstruction includejaundice, abdominal pain (reliably reported as right upperquadrant or epigastric pain in <strong>old</strong>er children and adolescents),nausea, and vomiting. A more acute presentationis seen when biliary obstruction is accompanied by cholangitis,which is a bacterial infection of the biliary tree causedby stasis of bile upstream from the obstruction. Patients afflictedwith cholangitis usually will have fever and leukocytosisand can develop bacterial sepsis.Gallstone DiseaseThe most common cause of biliary obstruction in <strong>old</strong>erchildren and adolescents is gallstone disease (termed cholelithiasis).Little is known about the epidemiology ofgallstone disease in pediatrics. The pigmented stone isthe most commonly identified type of gallstone in children;however, overweight adolescent girls are at particularrisk of developing cholesterol stones. Identified risk factorsfor the development of gallstones in children include hemolyticdisease, existing hepatobiliary disease, cystic fibrosis,Crohn disease, chronic PN exposure, and obesity. Ifa gallstone becomes lodged within the common bile duct(termed choledocholithiasis), obstructive jaundice will resultand anticipated laboratory findings include elevationsin conjugated bilirubin, alkaline phosphatase, and GGT.AST and ALT levels may or may not be elevated. Shouldthe gallstone impact distally at the junction of the commonbile duct and pancreatic duct, the patient may be symptomaticwith both obstructive jaundice and pancreatitis.Plain abdominal radiographs and computed tomographyare poor tests for the detection of gallstones becausemost stones are not calcified and therefore will not be visibleusing these techniques. Ultrasonography is highly sensitiveand specific for the detection of gallstones >1.5 mmin diameter within the lumen of the gallbladder; however,Pediatrics in Review Vol.33 No.7 July 2012 297
gastrointestinal disordersconjugated hyperbilirubinemiathe sensitivity drops off substantially for the detection ofgallstones within the common bile duct. The common bileduct will dilate in the setting of obstruction, and the diameterof the bile duct is readily measured by the ultrasonographer.The combination of a dilated common bile ductwith clinical and laboratory evidence of obstructive jaundiceis highly suspicious for a common bile duct stone.Many of these common bile duct stones will pass spontaneously,resulting in both clinical improvement in the patientand a decrease in the conjugated bilirubin level. Ifsymptoms persist, however, intervention is required urgentlybecause patients are at risk for development of cholangitisand bile duct perforation. Endoscopic retrograde cholangiopancreatography(ERCP) is the methodology of choice forthe investigation and treatment of common bile duct stones.ERCP can visualize the presence of the stone (Fig 4), andthen deploy a balloon catheter to sweep the stone out of thecommon bile duct. Typically, a sphincterotomy of the ampullaof Vater is performed to enlarge the opening of thecommon duct and allow for passage of the stone. Regardlessof whether or not a patient passes a common duct stonespontaneously or requires therapeutic intervention, all patientswho have symptomatic gallstone disease will requiresurgical cholecystectomy when clinically stable.Choledochal CystA CDC is a congenital anomaly of the biliary tract characterizedby cystic dilatation of some portion of the biliarytree. CDCs can present in the newborn period asconjugated hyperbilirubinemia and a palpable abdominalmass. Outside of the neonatal period, the classic triad ofsymptoms includes fever, right upper quadrant abdominalpain, and jaundice, symptoms that may be easily confusedwith gallstone disease. Stones and sludge can formin the dilated biliary tree, leading to obstruction and thedevelopment of cholangitis as well as pancreatitis. Thereare several anatomic subtypes of choledochal cyst, with themost common being type I, which represents cystic dilatationof the common bile duct.The diagnosis of CDC is made most often with abdominalultrasonography, which demonstrates a cystic massnear the porta hepatis that is in continuity with the biliarytree. For better anatomic definition and classification ofthe CDC, as well as for surgical planning, a more robustradiographic test often is desired. Previously, ERCP wasthe preferred method for visualization of the CDC; however,because of the risk of post-ERCP pancreatitis as wellas exposure to ionizing radiation, magnetic resonancecholangiopancreatography (MRCP) has replaced ERCPas the g<strong>old</strong> standard for characterization of CDCs.CDC is considered to be a premalignant state with aFigure 4. Contrast imaging of common bile duct via endoscopicretrograde cholangiopancreatography (ERCP) in a12-<strong>year</strong>-<strong>old</strong> girl who has right upper quadrant abdominalpain and conjugated hyperbilirubinemia. Arrows show fillingdefects in the lumen of a dilated common bile duct, representingmultiple gallstones in the common bile duct.significant lifetime risk of developing cholangiocarcinoma.Thus, for management of both acute biliary symptoms andfor cancer prevention, the treatment for a CDC is surgicalexcision and Roux-en-Y choledochojejunostomy.Other Causes of Obstructive JaundiceIn the setting of the acute onset of jaundice and fever,hydrops of the gallbladder should be suspected if abdominalultrasonography demonstrates a distended gallbladderwithout stones and with normal extrahepatic bileducts. Hydrops of the gallbladder in children is associatedwith Kawasaki disease as well as with acute streptococcaland staphylococcal infections.Tumors of the liver and biliary tract may present initiallywith jaundice, but jaundice rarely is an isolated finding andmore often is accompanied by abdominal pain and an abdominalmass. The possibility of tumor reinforces the importanceof the initial abdominal ultrasonography, whichwill suggest a mass, leading to subsequent computed tomographyor magnetic resonance imaging to evaluate thelesion further. Hepatic sinusoidal obstruction syndrome,previously referred to as hepatic veno-occlusive disease, isseen in both children and adults receiving treatment298 Pediatrics in Review Vol.33 No.7 July 2012
gastrointestinal disordersconjugated hyperbilirubinemiafor cancer, particularly hematologic malignancy. Throughmechanisms that are not fully understood, the developmentof microthrombi in hepatic sinusoids leads to hepaticdysfunction that often is severe. Patients present with jaundice,hepatomegaly, ascites, and laboratory evidence ofhepatic synthetic dysfunction in addition to conjugatedhyperbilirubinemia and elevation of aminotransferase levels.Diagnosis is suggested by abdominal ultrasonographywith Doppler measurement, which shows a decrease or reversalof portal venous blood flow.Infectious HepatitisThe acute onset of jaundice, typically associated with rightupper quadrant pain, hepatomegaly, nausea, and malaise,with variable fever, is suggestive of an infectious hepatitis.Elevation of AST and ALT levels, usually at least 2 to 3times the upper limit of normal, is always seen in an infectioushepatitis, although the degree of hyperbilirubinemiacan be variable. A broad range of viral agents can lead toinfectious hepatitis. The incidence of hepatitis A infectionin the United States has plummeted drastically since theuniversal implementation of vaccination. Both hepatitisB and hepatitis C can cause jaundice at the time of acuteinfection, and thus it is important to measure serologicmarkers for hepatitis A, B, and C viruses in any child oradolescent who have hepatitis. Epstein-Barr virus and cytomegalovirusboth can cause hepatitis and cholestasis inthe context of a mononucleosislike illness. Adenovirus, influenzavirus, parvovirus, members of the enterovirus family,herpes simplex virus, and varicella virus also can lead tohepatic involvement, usually in the context of other clinicalsymptoms typical of the individual virus.Autoimmune Disease of the Liverand Biliary SystemAutoimmune hepatitis (AIH) is characterized by a chronicactive hepatitis and non–organ-specific autoantibodies. (8)Without treatment, this chronic hepatitis progresses to cirrhosisand end-stage liver disease over time. AIH can presentat any age in children and adults, although the incidence increaseswith age during childhood and adolescence. AIH ismore common in girls, and the spectrum of clinical presentationis wide. AIH can present insidiously as fatigue, malaise,and recurrent fevers or fulminantly as acute liverfailure. Depending on the chronicity of disease, physicalfindings of portal hypertension may be present at diagnosis.Typically, there is elevation of AST and ALT levels, althoughwith considerable variation in the degree of elevation.Conjugated hyperbilirubinemia, a low albuminlevel, and an elevated prothrombin time indicate extensivechronic disease. The serum immunoglobulin G (IgG) levelusually is elevated, and 90% of patients will test positive forat least one of the associated autoantibodies: antinuclearantibody (ANA), anti–smooth muscle antibody (ASMA),and anti-liver, kidney microsomal antibody (LKM).Two distinct subtypes of AIH have been described andcan be distinguished by serologic autoantibody tests. Thefirst, AIH type I, is the most common, includes 80% of allpatients who have AIH, and is characterized by positiveANA or ASMA or both. AIH type 2 is more prevalentin younger children and is characterized by anti-LKM positivity.Children who have AIH type 2 are more likely topresent with acute hepatic failure. AIH can be associatedwith the autoimmune polyendocrinopathy-candidiasisectodermaldystrophy syndrome, one of the polyglandularautoimmune syndromes characterized by mucocutaneouscandidiasis, hypothyroidism, and adrenal insufficiency.Liver biopsy remains the g<strong>old</strong> standard in the diagnosisof AIH. Histologic features include a dense inflammatoryinfiltrate in the liver, consisting of mononuclear andplasma cells, that begins in the portal areas and extends beyondthe limiting plate into the parenchyma of the liver.Piecemeal necrosis of hepatocytes also is observed frequently.The treatment of AIH involves the use of immunosuppressiveagents. Conventionally, remission (definedas the normalization of AST and ALT levels) is inducedby using corticosteroids with a taper over several months.Corticosteroid-sparing agents, in particular the immunomodulatorazathioprine, are given long term to maintainremission of AIH.Primary sclerosing cholangitis (PSC) is a progressive,autoimmune-mediated disease targeting both the intraandextrahepatic bile ducts, resulting in significant scarringof the biliary tree. Patients present with laboratory evidenceof bile duct injury, having elevations of GGT andalkaline phosphatase levels. AST, ALT, and conjugated bilirubinconcentration may be elevated as well. Imaging ofbile ducts, either with MRCP or ERCP, shows evidence ofstricturing and dilation of affected portions of the biliarytree.PSC usually is associated with inflammatory bowel disease,particularly ulcerative colitis, and can progress slowlyto cirrhosis. Several features distinguish PSC in childrenfrom adult disease. (9) In a subgroup of children who havePSC, there is elevation of IgG levels, autoantibody titers,and histologic features on liver biopsy similar to AIH,known as “overlap syndrome.” These children may favorablyrespond to immunosuppressive therapy. However, formost children and adults with PSC, there is a disconcertinglack of immunomodulatory therapies that can reverse thecourse of PSC.Pediatrics in Review Vol.33 No.7 July 2012 299
gastrointestinal disordersconjugated hyperbilirubinemiaDrug- and Toxin-Induced CholestasisDrug hepatotoxicity can manifest in many forms, rangingfrom a systemic drug hypersensitivity syndrome to isolatedcholestasis. Although some forms of hepatotoxicity are predictable,most are idiosyncratic owing to genetic variabilityin drug metabolism, making it difficult to understand thepathogenesis of hepatotoxicity in a given patient. (10)Some drug-induced cholestasis can present as an isolatedelevation of conjugated bilirubin; however, often there isa mixed hepatitic-cholestatic reaction with elevation of aminotransferasesand conjugated bilirubin. Commonly usedmedications in pediatrics that potentially can present withcholestatic liver injury include amoxicillin/clavulanic acid,oral contraceptives, and erythromycin. In any patientwho has cholestasis of unknown origin, it is critical to obtaina comprehensive medication history that includes recreationaluse of drugs. Ecstasy, in particular, has been associatedwith hepatotoxicity and the development of jaundice. Usein adolescents of anabolic steroids for bodybuilding has beenreported to cause cholestasis. Questioning also should be directedat therapies that are not regulated by the Food andDrug Administration, such as nutritional supplements andhomeopathic treatments, because hepatotoxic metabolitesof these substances have been described.Wilson DiseaseWilson disease is caused by an autosomal recessive inheriteddefect in the ATP7B gene, which codes for a hepatocyteprotein responsible for trafficking of copper into bile.(<strong>11</strong>) If the liver cannot excrete copper, the metal accumulatesin the liver, brain, kidneys, and eyes. Copper toxicitythen produces the end-organ dysfunction seen in Wilsondisease. Wilson disease rarely presents before 5 <strong>year</strong>s ofage, but its age of presentation and clinical manifestationsvary. With age, the likelihood of liver involvement at presentationdecreases, whereas the likelihood of neuropsychiatricdisease increases.The spectrum of the hepatic presentation of Wilsondisease includes an acute syndrome with nausea, fatigue,and elevated aminotransferases, mimicking infectious hepatitis.Long-standing liver injury may present with jaundiceand conjugated hyperbilirubinemia. Other common hepaticpresentations of Wilson disease include chronic hepatitis,cirrhosis with portal hypertension, and fulminant hepaticfailure. A clue to Wilson disease in the laboratory evaluationis a low alkaline phosphatase level in the setting of elevationof serum aminotransferase and conjugated bilirubin levels.Wilson disease also can affect the kidneys, manifesting asproximal tubular dysfunction with urinary loss of uric acidand subsequent low serum uric acid levels. Wilson diseaseaffects the hematologic system, leading in some patients toa direct antibody test (Coombs)-negative hemolytic anemia.Because of the varied presentation of this disease,a high degree of suspicion for Wilson disease must be keptin every school-age child or adolescent presenting with anytype of hepatic injury.The practitioner must rely on interpretation of a numberof diagnostic studies in the evaluation for Wilson disease.The sensitivity and specificity of these tests can varydepending on the clinical presentation. Diagnostic testsinclude measurement of serum ceruloplasmin, which istypically low (40 mg issuggestive of the disorder. Liver tissue can be sent for quantitativecopper measurement, and genetic testing is available.Prompt diagnosis of Wilson disease is important because theinstitution of copper chelation therapy can halt progressionof the disease, which is uniformly fatal if untreated.Benign Recurrent Intrahepatic CholestasisAutosomal recessive mutations in canalicular transportproteins FIC1 and BSEP produce the phenotypes PFIC1and PFIC2, respectively, which typically present in infancyor childhood and may progress to liver failure early in life.Less severe mutations in these genes can produce the diseaseknown as benign recurrent intrahepatic cholestasis(BRIC). Importantly, BRIC does not lead to progressiveliver disease, cirrhosis, or hepatic dysfunction. BRIC is anepisodic disorder and presents in the first or second decadeafter birth with pruritis, often severe, and jaundice. Episodesmay be precipitated by viral illnesses and typicallyare heralded by the onset of pruritis, followed weeks laterby the development of jaundice. Nausea and steatorrheaalso may be present. Laboratory tests of liver function revealnormal or mildly elevated serum AST and ALT, withelevation of both conjugated bilirubin and alkaline phosphatase.The GGT concentration typically is normal ormildly elevated. The prothrombin time may be mildly prolongedbecause of vitamin K malabsorption and deficiencyin the setting of cholestasis. Episodes can last weeks tomonths, and patients are completely well with normal livertesting in the intermediary periods. Treatment is directedtoward relief of pruritis, typically with ursodeoxycholicacid and rifampin, and correction of any fat-soluble vitamindeficiencies.300 Pediatrics in Review Vol.33 No.7 July 2012
gastrointestinal disordersconjugated hyperbilirubinemiaWith the initial episode of pruritis and jaundice, anatomicand histologic tests may be required to distinguishBRIC from PSC and other causes of intrahepatic cholestasis.Detailed imaging of the biliary tree, with either ERCPor MRCP, will be normal. During an episode, the dominanthistologic finding in the liver is centrilobular cholestasis.Hepatic lobular or portal inflammation is anunusual finding in the liver. In contrast to other inflammatorydiseases of the liver, such as AIH and PSC, liverhistologyinBRICwillreturntonormalinasymptomaticperiods.Summary• A variety of anatomic, infectious, autoimmune, andmetabolic diseases can lead to conjugatedhyperbilirubinemia, both in the newborn period andlater in childhood.• The pediatric practitioner is most likely to encounterconjugated hyperbilirubinemia in the neonatalperiod.• It is crucial to maintain a high degree of suspicion forcholestasis in the persistently jaundiced newborn. Thegoal is recognition of conjugated hyperbilirubinemiabetween 2 and 4 weeks after birth, allowing for theprompt identification and management of infants whohave biliary atresia, which remains the most commoncause of neonatal cholestasis.References1. Davis AR, Rosenthal P, Escobar GJ, Newman TB. Interpretingconjugated bilirubin levels in newborns. J Pediatr. 20<strong>11</strong>;158(4):562–565. e12. Matte U, Mourya R, Miethke A, et al. <strong>An</strong>alysis of genemutations in children with cholestasis of undefined etiology.J Pediatr Gastroenterol Nutr. 2010;51(4):488–4933. Hartley JL, Davenport M, Kelly DA. Biliary atresia. Lancet.2009;374(9702):1704–17134. Lien TH, Chang MH, Wu JF, et al; Taiwan Infant Stool ColorCard Study Group. Effects of the infant stool color card screeningprogram on 5-<strong>year</strong> outcome of biliary atresia in Taiwan. Hepatology.20<strong>11</strong>;53(1):202–2085. Wagner M, Zollner G, Trauner M. New molecular insights intothe mechanisms of cholestasis. JHepatol. 2009;51(3):565–5806. Carter BA, Taylor OA, Prendergast DR, et al. Stigmasterol, a soylipid-derived phytosterol, is an antagonist of the bile acid nuclearreceptor FXR. Pediatr Res. 2007;62(3):301–3067. Strassburg CP. Hyperbilirubinemia syndromes (Gilbert-Meulengracht,Crigler-Najjar, Dubin-Johnson, and Rotor syndrome). Best PractRes Clin Gastroenterol. 2010;24(5):555–5718. Mieli-Vergani G, Heller S, Jara P, et al. Autoimmune hepatitis.J Pediatr Gastroenterol Nutr. 2009;49(2):158–1649. Mieli-Vergani G, Vergani D. Unique features of primarysclerosing cholangitis in children. Curr Opin Gastroenterol. 2010;26(3):265–26810. Navarro VJ, Senior JR. Drug-related hepatotoxicity. N EnglJ Med. 2006;354(7):731–739<strong>11</strong>. Roberts EA, Schilsky ML; Division of Gastroenterology andNutrition, Hospital for Sick Children, Toronto, Ontario, Canada.A practice guideline on Wilson disease. Hepatology. 2003;37(6):1475–1492Suggested ReadingSuchy FJ, Sokol RJ, Balistreri WF, eds. Liver Disease in Children.3rd ed. New York, NY: Cambridge University Press; 2007PIR QuizThis quiz is available online at http://www.pedsinreview.aappublications.org. NOTE: Since January 2012, learners cantake Pediatrics in Review quizzes and claim credit online only. No paper answer form will be printed in the journal.New Minimum Performance Level RequirementsPer the 2010 revision of the American Medical Association (AMA) Physician’s Recognition Award (PRA) and creditsystem, a minimum performance level must be established on enduring material and journal-based CME activities thatare certified for AMA PRA Category 1 Credit TM . To successfully complete 2012 Pediatrics in Review articles for AMAPRA Category 1 Credit TM , learners must demonstrate a minimum performance level of 60% or higher on thisassessment, which measures achievement of the educational purpose and/or objectives of this activity.Starting with 2012 Pediatrics in Review, AMA PRA Category 1 Credit TM can be claimed only if 60% or more of thequestions are answered correctly. If you score less than 60% on the assessment, you will be given additionalopportunities to answer questions until an overall 60% or greater score is achieved.Pediatrics in Review Vol.33 No.7 July 2012 301
gastrointestinal disordersconjugated hyperbilirubinemia1. A 3-month-<strong>old</strong> boy is jaundiced and is found to have conjugated hyperbilirubinemia; however, his gammaglutamyltransferase level is in the low normal range. Which of the following conditions is most likely tobe present?A. Alpha-1 antitrypsin deficiencyB. Biliary atresiaC. Cystic fibrosisD. Progressive familial intrahepatic cholestasisE. Rubella infection2. A 14-<strong>year</strong>-<strong>old</strong> girl presents with a history of intermittent right upper quadrant pain over the last 2 months. Herlaboratory evaluation reveals a direct bilirubin of 2.3 mg/dL. Of the following, what is the most appropriatenext study?A. Abdominal ultrasonographyB. Endoscopic retrograde cholangiopancreatographyC. Hepatobiliary iminodiacetic acid scanD. Liver biopsyE. Targeted mutation analysis of the uridine diphosphate glucuronosyltransferase 1-1 gene to assess forGilbert syndrome3. You are examining a jaundiced 1-month-<strong>old</strong> girl and hear a heart murmur consistent with peripheral pulmonicstenosis. A blood test reveals conjugated hyperbilirubinemia, causing you to suspect this condition:A. Alagille syndromeB. Biliary atresiaC. Cystic fibrosisD. HypothyroidismE. Progressive familial intrahepatic cholestasis4. A toddler who has chronic cholestasis has pruritus that is refractory to ursodeoxycholic acid. Which of thefollowing medications may be helpful in reducing symptoms?A. AmoxicillinB. DiphenhydramineC. OndansetronD. RifampinE. Sulfisoxazole5. A 5-week-<strong>old</strong> boy has been found to have biliary atresia. His parents are hesitant to authorize surgery andprefer “to see how he progresses. If he does not do well, he can always have surgery later.” Which of thefollowing statements regarding Kasai portoenterostomy is true:A. Age at the time of the Kasai procedure is not associated with surgical outcome.B. Approximately 50% of children with biliary atresia have spontaneous resolution of their disease and do notrequire a Kasai procedure.C. The Kasai procedure involves insertion of an prosthetic bile duct.D. The Kasai procedure is curative and most patients do not require follow-up of their liver disease.E. The Kasai procedure, when performed at
Articlecollagen vascular disordersJuvenile Idiopathic ArthritisMaria Espinosa, MD,*Beth S. Gottlieb, MD, MS*Author DisclosureDrs Espinosa andGottlieb havedisclosed no financialrelationships relevantto this article. Thiscommentary does notcontain a discussion ofan unapproved/investigative use ofa commercial product/device.Educational GapJuvenile idiopathic arthritis affects around 294,000 children in the United States. In 2001,a new classification of the disorder and its subtypes was created. Current therapies, includingthe use of biologic medications, have improved the prognosis of this condition significantly.Objectives After completing this article, readers should be able to:1. Understand the pathophysiology of juvenile idiopathic arthritis (JIA).2. Recognize the clinical features of the different types of JIA.3. Be aware of the complications of JIA.4. Know the treatment of JIA.IntroductionJuvenile idiopathic arthritis (JIA) is a broad term used to describe several different forms ofchronic arthritis in children. All forms are characterized by joint pain and inflammation.The <strong>old</strong>er term, juvenile rheumatoid arthritis, has been replaced by JIA to distinguish childhoodarthritis from adult-onset rheumatoid arthritis and to emphasize the fact that arthritisin childhood is a distinct disease. JIA also includes more subtypes of arthritis than did juvenilerheumatoid arthritis.JIA is the most common rheumatologic disease in children and is one of the more frequentchronic diseases of childhood. The etiology is not completely understood but is known to bemultifactorial, with both genetic and environmental factors playing key roles. Without appropriateand early aggressive treatment, JIA may result in significant morbidity, such as leg-lengthdiscrepancy, joint contractures, permanent joint destruction, or blindness from chronic uveitis.DefinitionArthritis is defined as joint effusion alone or the presence of two or more of the followingsigns: limitation of range of motion, tenderness or pain on motion, and increased warmth inone or more joints. JIA is broadly defined as arthritis of one or more joints occurring for atleast 6 weeks in a child younger than 16 <strong>year</strong>s of age. JIA isa diagnosis of exclusion. A number of conditions, such asAbbreviationsinfections, malignancy, trauma, reactive arthritis, and connectivetissue diseases such as systemic lupus erythematosusANA: antinuclear antibody(SLE), must be excluded before a diagnosis of JIA can beARF: acute rheumatic fevermade (1) (Table 1).AS: ankylosing spondylitisJIA is subdivided into seven distinct subtypes in the classificationscheme established by the International League ofIBD: inflammatory bowel diseaseIL: interleukinAssociations for Rheumatology in 2001 (Table 2). The subtypesdiffer according to the number of joints involved, pat-IV: intravenousJIA: juvenile idiopathic arthritistern of specific serologic markers, and systemic manifestationsMAS: macrophage activation syndromepresent during the first 6 months of disease. These categoriesNSAID: nonsteroidal anti-inflammatory drugwere established to reflect similarities and differences amongRF: rheumatoid factorthe different subtypes so as to facilitate communicationSLE: systemic lupus erythematosusamong physicians worldwide, to facilitate research, and toTNF: tumor necrosis factoraid in understanding prognosis and therapy. (2)*The Steven and Alexandra Cohen Children’s Medical Center of New York, North Shore Long Island Jewish Health System, NewHyde Park, NY.Downloaded from http://pedsinreview.aappublications.org/ by Enrique Mendoza-Lopez on July 2, 2012Pediatrics in Review Vol.33 No.7 July 2012 303
collagen vascular disordersjuvenile idiopathic arthritisTable 1. Differential Diagnosis ofArthritisReactiveInflammatoryInfectionSystemicMalignancyBenign bone tumorsImmunodeficiencyTraumaPoststreptococcalRheumatic feverSerum sickness“Reiter syndrome”Juvenile idiopathic arthritisInflammatory bowel diseaseSarcoidosisSeptic jointPostinfectious: toxicsynovitisViral (eg, Epstein-Barr virus,parvovirus)Lyme diseaseOsteomyelitisSacroilitis, bacterialDiscitisSystemic lupuserythematosusHenoch-Schönlein purpuraSerum sicknessDermatomyositisMixed connective tissuediseaseProgressive systemic sclerosisPeriodic fever syndromesPsoriasisKawasaki diseaseBehçet diseaseLeukemiaNeuroblastomaMalignant bone tumors (eg,osteosarcoma, Ewingsarcoma, rhabdosarcoma)Osteoid osteomaOsteoblastomaCommon variableimmunodeficiencyAdapted from Weiss JE, Illowite NT. Juvenile idiopathic arthritis.Rheum Dis Clin N Am. 2007;33:441–470.EpidemiologyIt has been estimated that JIA affects w294,000 childrenbetween the ages of 0 and 17 <strong>year</strong>s in the United States.The incidence and prevalence of JIA vary worldwide.This difference likely reflects specific genetic (eg, HLAantigen alleles) and environmental factors in a given geographicarea. The incidence rate has been estimated as 4to 14 cases per 100,000 children per <strong>year</strong>, and the prevalencerates have been reported as 1.6 to 86.0 cases per100,000 children. JIA tends to occur more frequently inchildren of European ancestry, with the lowest incidencerates reported among Japanese and Filipino children.In white populations with European ancestries, oligoarticularJIA is the most common subtype. In children ofAfrican American descent, however, JIA tends to occurat an <strong>old</strong>er age and is associated with a higher rate ofrheumatoid factor (RF)-positive polyarticular JIA anda lower risk of uveitis.Different subtypes of JIA vary with respect to age andgender distributions (Table 2). Oligoarticular JIA, forexample, occurs more frequently in girls, with a peak incidencein children between 2 and 4 <strong>year</strong>s of age. PolyarticularJIA also occurs more frequently in girls and hasa biphasic age of onset; the first peak is from 1 to 4 <strong>year</strong>sof age and the second peak occurs at 6 to 12 <strong>year</strong>s of age.(1)(3)PathogenesisThe cause of JIA is not well understood, but is believed tobe influenced by both genetic and environmental factors.Twin and family studies strongly support a genetic basisof JIA; concordance rates in monozygotic twins rangebetween 25% and 40%, and siblings of those affectedby JIA have a prevalence of JIA that is 15- to 30-f<strong>old</strong>higher than the general population.Strong evidence has been reported for the role of HLAclass I and II alleles in the pathogenesis of different JIAsubtypes. HLA-B27 has been associated with the developmentof inflammation of the axial skeleton with hip involvement,and often is positive in patients who haveenthesitis-related arthritis. HLA-A2 is associated withearly-onset JIA. The class II antigens (HLA-DRB1*08,<strong>11</strong>, and 13 and DPB1*02) are associated with oligoarticularJIA. HLA-DRB1*08 is also associated with RFnegativepoly JIA.Clinical features of systemic-onset JIA mostly resembleautoinflammatory syndromes, such as familial Mediterraneanfever, and there is a lack of an association betweensystemic-onset JIA and HLA genes. As a result, many concludethat systemic-onset JIA should be considered a separateentity, distinct from the other JIA subtypes. (4)Cell-mediated and humoral immunity play a role inthe pathogenesis of JIA. T cells release proinflammatorycytokines, such as tumor necrosis factor a (TNF-a),interleukin-6(IL-6),andIL-1,whicharefoundinhighlevelsin patients who have polyarticular JIA and systemiconsetJIA. Evidence for the role of T cells in JIA comesfrom studies that show oligoclonal expansion of T cells anda high percentage of activated T cells in the synovium ofpatients who have JIA.304 Pediatrics in Review Vol.33 No.7 July 2012Downloaded from http://pedsinreview.aappublications.org/ by Enrique Mendoza-Lopez on July 2, 2012
collagen vascular disordersjuvenile idiopathic arthritisTable 2. International League of Associations for Rheumatology Classification of JuvenileIdiopathic ArthritisCategory DefinitionFrequency(% of all JIA) Age of Onset Sex RatioSusceptibilityAllelesSystemic onsetjuvenileidiopathicarthritis (JIA)Arthritis in one or more joints with or precededby fever of at least 2 weeks’ duration that isdocumented as daily (“quotidian”) for at least3 days and accompanied by one or more of thefollowing: (1) rash (evanescent), (2)lymphadenopathy, (3) hepatomegaly orsplenomegaly, (4) serositisOligo JIA Arthritis affecting one to four jointsduring the first 6 months of disease• Persistent Affects no more than four jointsthroughout the disease course• Extended Affects more than four joints after thefirst 6 months of diseasePolyarthritis Arthritis affects five or more joints in the(RF-negative) first 6 months of disease. Tests for RFare negativePolyarthritis(RF-positive)Arthritis affects five or more joints in thefirst 6 months of disease. Tests for RF arepositive on at least two occasions that are3 months apartPsoriatic arthritis Arthritis and psoriasis, or arthritis and at leasttwo of the following: (1) dactylitis, (2) nailpitting, (3) family history of psoriasis in afirst-degree relativeEnthesitis-relatedarthritisUndifferentiatedarthritisArthritis or enthesitis with at least two of thefollowing: (1) sacroiliac tenderness orlumbosacral pain, (2) presence of HLA-B27antigen, (3) onset of arthritis in a male >6<strong>year</strong>s <strong>old</strong>, (4) acute anterior uveitis,(5) family history in a first-degree relativeof HLA-B27–associated diseaseArthritis that fulfills criteria in no categoryor in two or more of the above categories4%–17% Childhood F[M HLA-DRB1*<strong>11</strong>27%–56% Early childhood;peak at 2–4 <strong>year</strong>s<strong>11</strong>%–28% Biphasic distribution;early peak at 2–4 <strong>year</strong>sand later peak at 6–12<strong>year</strong>s2%–7% Late childhood oradolescence2%–<strong>11</strong>% Biphasic distribution;early peak at 2–4 <strong>year</strong>sand later peak at 9–<strong>11</strong><strong>year</strong>s3%–<strong>11</strong>% Late childhood oradolescence<strong>11</strong>%–21%F>>>M HLA-DRB1*08HLA-DRB1*<strong>11</strong>HLA-DQA1*04HLA-DQA1*05HLA-DQB1*04HLA-A2 (earlyonset)F>>M HLA-DRB1*0801F>>M HLAB1*04HLA-DR4F>M HLA-B27IL23R † (newassociation)M>>F HLA-B27ERAP1 † (newassociation)Adapted from Ravelli A, Martini A. Juvenile idiopathic arthritis. Lancet. 2007;369:767–768.† Hinks A, Martin P, Flynn E, et al. Subtype specific genetic associations for JIA: ERAP1 with the enthesitis related arthritis subtype and IL23R with juvenile psoriatic arthritis. Arthritis Res Ther. 20<strong>11</strong>;13:R12. HLA¼human lymphocyte antigen; JIA¼juvenile idiopathic arthritis; RF¼rheumatoid factor.Downloaded from http://pedsinreview.aappublications.org/ by Enrique Mendoza-Lopez on July 2, 2012Pediatrics in Review Vol.33 No.7 July 2012 305
collagen vascular disordersjuvenile idiopathic arthritisRecently, inflamed joints in patients who have JIAhave been shown to have high levels of IL-17–producingT cells; IL-17 induces the production of other interleukinsand matrix metalloproteinases that are all involvedin joint damage. The role of humoral immunity in JIApathogenesis is supported by the increased level of autoantibodies,such as antinuclear antibodies (ANAs) andimmunoglobulins, by complement activation, and by thepresence of circulating immune complexes. (5)Other possible factors that have been implicated in thepathogenesis of JIA include immunologic dysregulation,psychological stress, trauma, hormonal abnormalities, andinfectious triggers.Clinical FeaturesJIA is divided into seven subtypes defined by clinical featuresduring the first 6 months of disease. The InternationalLeague of Associations for Rheumatology classification ofJIA includes the following subtypes: (1) Systemic-onsetarthritis, (2) oligoarticular arthritis, (3) polyarticular RFpositivearthritis, (4) polyarticular RF-negative arthritis,(5) psoriatic arthritis, (6) Enthesitis-related arthritis, and(7) undifferentiated arthritis, or “other.” Each subtypevaries with respect to clinical presentation, pathogenesis,treatment outcomes, and prognosis. All subtypes of JIA,however, share common symptoms, such as morningstiffness or “gelling phenomenon” (stiffness after a jointremains in one position for a prolonged period) thatimproves throughout the day, limp, swollen joints, limitationof activities because of pain, and periods characterizedby disease remission interspersed with disease flares.There is no diagnostic test for JIA; therefore, othercauses of arthritis must be excluded carefully before thediagnosis is made.The rash in systemic JIA is described typically as an evanescent,salmon-colored macular rash that accompaniesfebrile periods (Fig 1). The rash generally is nonpruriticand occurs most commonly on the trunk and proximalextremities, including the axilla and inguinal areas. (2)Other extra-articular manifestations that can be seen insystemic JIA include hepatosplenomegaly, lymphadenopathy,pulmonary disease, such as interstitial fibrosis,and serositis, such as pericarditis. The febrile period andother systemic features may precede the onset of arthritisby weeks to months. A definite diagnosis of JIA, however,cannot be made until arthritis is detected on physical examination.(6)Laboratory abnormalities typically observed in systemicJIA include anemia, leukocytosis, thrombocytosis,elevated liver enzymes, and acute-phase reactants, such aserythrocyte sedimentation rate, C-reactive protein, andferritin. ANA titer is usually negative and is not helpfulin making the diagnosis.Complications of systemic JIA include infection fromimmunosuppressive therapy, growth disturbances, osteoporosis,cardiac disease, amyloidosis (rare in NorthAmerica compared with other parts of the world), andmacrophage-activation syndrome (MAS) (Table 3). MASoccurs in about 5% to 8% of children who have systemicJIA and is characterized by persistent fever, pancytopenia,hepatosplenomegaly, liver dysfunction, coagulopathy,and neurologic symptoms. Bone marrow examinationSystemic-Onset JIASystemic-onset JIA is distinct compared with the othersubtypes in that it is characterized by the presence ofhigh-spiking fevers of at least 2 weeks’ duration in additionto arthritis. The disease affects 10% to 15% ofchildren who have JIA, and tends to affect boys andgirls equally, with a peak age of onset between 1and 5 <strong>year</strong>s. Early in the disease course, patients canpresent with fatigue and anemia. The fever in systemicJIA is characterized by temperatures >39°C that occurdaily or twice daily, with a rapid return to baseline or belowbaseline (quotidian pattern). Fever spikes usually occur inthe late afternoon or evening. Children often appear illduring febrile periods and look well when the feversubsides.Figure 1. Salmon-colored rash in systemic juvenile idiopathicarthritis. (Courtesy of Charles H. Spencer [http://www.rheumatlas.org].)306 Pediatrics in Review Vol.33 No.7 July 2012Downloaded from http://pedsinreview.aappublications.org/ by Enrique Mendoza-Lopez on July 2, 2012
collagen vascular disordersjuvenile idiopathic arthritisTable 3. Macrophage ActivationSyndromePhysical findingsLaboratory findingsBone marrowTreatmentBruising, purpura, mucosalbleedingEnlarged lymph nodes,enlarged liver and spleenElevated: AST, ALT, PT, PTT,fibrin degradation products,ferritin, triglyceridesDecreased: white blood cell andplatelet counts, erythrocytesedimentation rate,fibrinogen, clotting factorsActive phagocytosis bymacrophages and histiocytesIntravenous glucocorticoid,cyclosporineALT¼alanine aminotransferase; AST¼aspartate aminotransferase; PT¼prothrombin time; PTT¼partial thromboplastin.Adapted from Cassidy JT, Petty RE, Laxer RM, Lindsley CB. Textbook ofPediatric Rheumatology. 5th ed. Philadelphia, PA: Elsevier Inc.; 2005.in patients who have MAS reveals phagocytosis of hematopoieticcells by macrophages. (2) Triggers of MAS includeviral infections and certain changes in medications.Laboratory abnormalities include pancytopenia, prolongationof the prothrombin time and partial thromboplastintime, and elevated levels of D-dimer, triglycerides,and ferritin. Contrary to what would be expected, theerythrocyte sedimentation rate typically falls in MAS becauseof the low fibrinogen levels resulting from a consumptioncoagulopathy and hepatic dysfunction. Because MAScarries a significant mortality rate of approximately 20% to30%, early recognition and treatment of MAS with corticosteroidsor cyclosporine is important to prevent multisystemorgan failure. (6)Diagnosis of systemic JIA involves the exclusion ofother conditions, such as infections, malignancy, collagenvascular diseases, and acute rheumatic fever (ARF). Infectionstend to have less-predictable fever patterns than systemicJIA. Children who have leukemia tend to haveleukopenia, thrombocytopenia, and elevated lactic dehydrogenaselevels. In ARF, the fever tends to be persistent,the arthritis is migratory and asymmetric, cardiac involvementoften is associated with endocarditis, and the rash(referred to as erythema marginatum) is associated withan expanding margin. <strong>An</strong>tistreptolysin O titers can be elevatedin any inflammatory condition; however, the morespecific antibodies for streptococcal infection, such asantideoxyribonuclease b, antistreptokinsase, and antihyaluronidase,would be elevated only in ARF, indicatinga recent group A streptococcal infection.The prognosis of systemic JIA depends on the severityof the arthritis. Most systemic symptoms resolve overmonths to <strong>year</strong>s, and mortality, which is
collagen vascular disordersjuvenile idiopathic arthritisgrowth plate at sites of inflammation, which leads to overgrowth.This complication is most common with knee arthritisand it leads to a leg length discrepancy. Later in thedisease course, growth disturbances can result also fromgrowth plate damage or premature fusion of the epiphysealplates, leading to undergrowth of an affected extremity. (6)One of the most serious complications of JIA is iritis.Approximately 15% to 20% of children who have oligoarticularJIA are found to have iritis. The iritis tends tobe a chronic, anterior, nongranulomatous inflammationaffecting the iris and ciliary body and often is asymptomatic.This complication tends to occur in girls affectedwith oligoarticular JIA at a young age who have positiveANA titers. Appropriate ophthalmologic screening evaluationis imperative in all children who have JIA, especiallythose who have oligoarticular JIA and are ANA-positive(Table 4). If left untreated, complications include cornealclouding, cataracts, band keratopathy, synechiae, glaucoma,and visual loss (Fig 3). The outcome depends onearly diagnosis and treatment. (2)The differential diagnosis of a child with oligoarthritisincludes trauma, septic arthritis, Lyme disease, postinfectiousarthritis, and malignancy. In a child who presentswith features of an infectious illness, synovial fluid analysisand cultures are important to distinguish inflammatoryfrom infectious processes. In a septic joint, for example,there usually are more than 100,000 white blood cells/mm 3 , with 90% being polymorphonuclear neutrophils.Lyme arthritis can occur weeks to months after the initialinfection, and children typically will present with acuteonset of a large, swollen joint, typically the knee.In children who have oligoarticular JIA, laboratoryevaluation may be normal or indicate a mild increase ininflammatory markers. Tests for RF often are negative,and tests for ANA may be positive in low titers in 70%to 80% of children who have oligoarthritis, especially girlsand those who have iritis. (2)Among children who have JIA, those with oligoarthritishave the best prognosis. Children who develop a morecomplicated disease, characterized by joint space narrowing,bone erosions, and flexion contractures, are more likelyto be those who have a polyarticular course.Polyarticular JIAChildren affected by arthritis in five or more joints duringthe first 6 months of disease are diagnosed as having polyarticularJIA. Polyarticular JIA can be either RF-positive(seropositive) or RF-negative (seronegative). RF-positivedisease affects approximately 5% to 10% of patients whohave JIA and mainly affects girls in late childhood or earlyadolescence. Seropositive patients tend to develop an arthritissimilar to adult rheumatoid arthritis, having a moreaggressive disease course. There tends to be symmetric,small joint involvement of both the hands and feet andthe cervical spine and temporomandibular joints alsomay be affected (Fig 4). Rheumatoid nodules and a moresevere erosive disease characterized by joint deformities(ie, Boutonnière and Swan neck contractures) also mayoccur in patients who are RF-positive. (1) Patients withRF-negative arthritis tend to have involvement of fewerjoints and have a better overall functional outcome.Children who have polyarticular JIA may present withmorning stiffness, joint swelling, and limited range ofmotion of the affected joints. In addition, they also mayexperience fatigue, growth disturbances, elevated inflammatorymarkers, and anemia of chronic disease. Iritis maydevelop, although less frequently than in patients whohave oligoarticular disease.The differential diagnosis of patients presenting withpolyarthritis includes infection, malignancy, and othercollagen vascular diseases such as SLE. Polyarthritis inan adolescent girl could be an initial manifestation ofSLE; serologic tests for lupus must be sent.Table 4. American Academy of Pediatrics Guidelines for Screening EyeExaminationsJuvenile Idiopathic Arthritis (JIA) Subtype Risk of Iritis Examination FrequencyOligoarticular or polyarticular, onset 7 <strong>year</strong>s of age regardless of antinuclear antibody Medium riskEvery 6 monthsstatusSystemic onset JIA Low risk Every 12 monthsAdapted from Ravelli A, Martini A. Juvenile idiopathic arthritis. Lancet. 2007; 369:767–768.308 Pediatrics in Review Vol.33 No.7 July 2012Downloaded from http://pedsinreview.aappublications.org/ by Enrique Mendoza-Lopez on July 2, 2012
collagen vascular disordersjuvenile idiopathic arthritisFigure 3. Cataracts resulting from chronic uveitis in a patientwith juvenile idiopathic arthritis.Psoriatic ArthritisJuvenile psoriatic arthritis is characterized as an asymmetricarthritis that can affect both large and small joints andtypically has an onset in mid childhood. The condition isdefined more specifically by the presence of arthritis andthe typical psoriatic rash, or any two of the following if therash is absent: family history of psoriasis in a first-degreerelative, dactylitis (diffuse swelling of fingers extending beyondthe joint margin), and nail pitting (Fig 5). Childrenwho have psoriatic arthritis may develop iritis and shouldtherefore undergo slit-lamp evaluations every 6 months.These children also may be found to be ANA-positiveand HLA-B27–positive, especially when there is inflammationof the axial skeleton. (1)Enthesitis-Related ArthritisChildren affected by enthesitis-related arthritis generallyare boys >8 <strong>year</strong>s of age. This type of arthritis isFigure 4. Proximal interphalangeal joint and metacarpalphalangealjoint swelling (see thumbs) typical of polyarticularjuvenile idiopathic arthritis. (Courtesy of Charles H. Spencer[http://www.rheumatlas.org].)Figure 5. Swelling of left third proximal phalangeal joint with“sausage” appearance of finger in a patient with psoriatic arthritis.(Courtesy of Charles H. Spencer [http://www.rheumatlas.org].)characterized by the presence of enthesitis, or inflammationat the sites of tendon insertions onto bone. Most patientsafflicted with this type of arthritis are HLA-B27–positive. Patients typically complain of pain, stiffness,and loss of mobility of the lower back, and can presentwith arthritis in lower extremity joints. Unlike other JIAsubtypes, the sacroiliac joints can be involved at presentation.Children with this subtype may experience anterioror acute iritis, which is characterized by injected,erythematous conjunctiva, photophobia, and pain. Manypatients who have this type of arthritis have a positivefamily history of an HLA-B27–related disease, such asIBD, psoriasis, or ankylosing spondylitis (AS). (2)Patients who have enthesitis-related arthritis may developAS, reactive arthritis, or IBD-associated arthritis. Childrenwho have AS typically present with limitation and painof the lumbar spine and may have evidence of sacroiliacjoint inflammation on imaging. AS is most common inboys, with a male-to-female ratio of 7:1, and 90% of patientsare found to be positive for HLA-B27. Reactive arthritis oftenoccurs after a genitourinary or gastrointestinal infectionand often is associated with conjunctivitis and urethritis. PatientswhohaveIBDmaypresentinitiallywithanasymmetricarthritis involving joints of the lower extremities. Flaresof IBD also may be associated with episodic arthritis. (1)Undifferentiated ArthritisChildren diagnosed as having an undifferentiated arthritisgenerally do not meet inclusion criteria for any other category,or they may meet criteria for more than one. (2)Downloaded from http://pedsinreview.aappublications.org/ by Enrique Mendoza-Lopez on July 2, 2012Pediatrics in Review Vol.33 No.7 July 2012 309
collagen vascular disordersjuvenile idiopathic arthritisComplicationsOne of the more common and devastating complicationsassociated with JIA is iridocyclitis, a form of chronic anterioruveitis. The condition occurs in approximately 15%to 20% of patients who have JIA and can lead to permanentblindness. (6) It is critical that children who have JIA bescreened routinely for iritis because the uveitis can be diagnosedearly in the course only with a slit lamp examinationby an ophthalmologist. The frequency of required examinationsis determined by the child’s age and his or herANA status. Children 20 mg/d) and includeimmunosuppression, adrenal suppression, increasedappetite and weight gain, acne, mood changes, osteoporosisand avascular necrosis, cataracts and increased intraocularpressures, cushingoid features, and diabetes. (2)Disease-modifying antirheumatic drugs are agentsthat slow the radiologic progression of disease andare required by two-thirds of children. These agentsinclude sulfasalazine, azathioprine, hydroxychloroquine,leflunomide, cyclosporin, and methotrexate. Methotrexate,a folate antagonist, is the disease-modifying antirheumaticdrug most commonly prescribed in children whohave more aggressive arthritis. Methotrexate is givenonce weekly in either the oral or subcutaneous route.The effects of this medication generally are seen within6 to 12 weeks. Adverse effects mainly include gastrointestinalmanifestations, such as oral ulcers, abdominal pain,nausea, decreased appetite, and hepatic dysfunction(ie, elevation of liver enzymes). Folic acid can be administeredto decrease these gastrointestinal side effects.Pulmonary toxicity is a known adverse effect thatrarely occurs in children. There is an increased risk of310 Pediatrics in Review Vol.33 No.7 July 2012Downloaded from http://pedsinreview.aappublications.org/ by Enrique Mendoza-Lopez on July 2, 2012
collagen vascular disordersjuvenile idiopathic arthritisimmunosuppression while on methotrexate and patientsshould not receive any live virus vaccines such as measlesmumps-rubella,varicella, and intranasal flu vaccines. A childtaking methotrexate who develops a fever or is unwellshould be examined by the pediatrician and have studiessent (complete blood count, blood and urine cultures) toexclude an underlying bacterial infection. <strong>An</strong> increased riskof lymphoproliferative malignanciesalsoisreportedinchildrenwho take methotrexate, but this effect has not beenproven clearly. Blood counts and liver enzymes are monitoredevery 4 to 8 weeks while a child is taking methotrexate.The treatment period is not defined clearly, butgenerally, a child is treated with methotrexate for at least1 <strong>year</strong> after achieving disease remission. Overall, methotrexateis a very safe and effective drug and is now considereda “g<strong>old</strong>-standard” therapy for children who have JIA. (2)(8)Use of biologic agents has improved the morbidity associatedwith JIA significantly. Biologic drugs are medications,such as monoclonal antibodies, soluble cytokinereceptors, and receptor antagonists, that target specificproteins involved in the inflammatory cascade. All biologicsare given through the IV or subcutaneous route. Allof these agents carry a risk of immunosuppression andcytopenias; therefore, a child taking a biologic agent mustbe followed closely with detailed physical examinationsand laboratory studies.As with methotrexate, a child taking a biologic who developsa fever or appears unwell even without a fever (biologicssuch as anti-TNFs can block the febrile responsedespite active infection) must be examined and have bloodwork to exclude a serious bacterial infection. Biologicsshould not be given while a child is acutely ill. Also, childrenon biologics should not be given live vaccines. Reactivationof tuberculosis is another potential complication, and patientsare screened for tuberculosis before the start of therapyand then <strong>year</strong>ly while on these medications. (8)Elevated levels of TNF-a are found in patients who haveJIA. Etanercept, infliximab, and adalimumab are biologicagents that block TNF-a. Etanercept is a soluble TNF receptorthat binds and inhibits TNF-a and was approved bythe FDA in 1999 for the treatment of JIA in children >2<strong>year</strong>s of age. The drug has been shown to be highly effectivein patients who have extended oligoarthritis or polyarticularJIA who were not responsive to treatment with NSAIDs ormethotrexate. In addition to the risk of immunosuppression,headache, upper respiratory tract infections, and injectionsite reactions are other common adverse effects.Infliximab, a chimeric monoclonal antibody to TNF-a thatis given through the IV route, has been shown to be efficaciousin the treatment of JIA and uveitis. Adalimumab,a humanized monoclonal antibody to TNF, was thesecond biologic agent to be approved by the FDA in2008 for moderate to severe JIA in children >4 <strong>year</strong>sof age. Unlike etanercept, which is given once weekly,adalimumab is given once every 2 weeks and has beenshown to be effective in patients who have polyarticular JIA.Elevated levels of IL-1 and IL-6 are found in the seraand synovial fluid of patients who have JIA. These levelsare particularly elevated in children who have systemic-onsetJIA. Recently, anakinra, an anti-IL-1 receptor antagonist,and tocilizumab, an anti-IL-6 monoclonal antibody, whichis now approved by the FDA, have demonstrated promisingresults in the treatment of patients who have systemic JIA.Abatacept, a recombinant fusion protein that down-regulatesT-cell stimulation, was approved by the FDA in 2008 formoderate to severe polyarticular JIA in children >6 <strong>year</strong>s<strong>old</strong>. Other therapies, such as rituximab (an anti-CD20B-cell–depleting monoclonal antibody) and rilonacept (anIL-1 blocking agent), are being studied for the treatmentof JIA. The duration of treatment with biologics is at leastfor 1 <strong>year</strong> after disease remission is achieved. (2)(7)(8)Treatment of uveitis depends largely on the ophthalmologist’srecommendations. Typically, dilating agentsand topical corticosteroids are used first. If inflammationpersists or the patient is unable to taper off corticosteroidophthalmic drops, often methotrexate is started. Infliximabandadalimumabalsohavebeenfoundtobequitebeneficialin the treatment of uveitis. (9)Autologous Stem Cell TransplantationPatients who have JIA that is refractory to the previouslydescribed medical interventions may undergo autologousstem cell transplantation. Autologous stem cell transplantationinvolves using immunosuppression to removeautoreactive lymphocytes followed by stem cell transplantation.This procedure would be considered onlyfor a small subset of patients who have JIA that is refractoryto all other treatments. (7)Other ConsiderationsOther treatment considerations must include physical therapyand occupational therapy to improve mobility of affectedjoints and maintain muscle strength. Monitoringphysical and psychological functioning must be assessedroutinely, and counseling or psychotherapy offered whenneeded. <strong>Leg</strong>-length discrepancies may require treatmentif they become significant and orthopedic referrals shouldbe made when appropriate.PrognosisApproximately 50% of children who have JIA continue tohave active disease into adulthood. In patients who haveDownloaded from http://pedsinreview.aappublications.org/ by Enrique Mendoza-Lopez on July 2, 2012Pediatrics in Review Vol.33 No.7 July 2012 3<strong>11</strong>
collagen vascular disordersjuvenile idiopathic arthritisactive disease into adulthood, there can be significant disability,such as joint deformity, growth abnormalities, visualdisturbance caused by uveitis, functional limitationsbecause of pain, and so forth. Factors affecting diseaseoutcome include disease duration, presence of polyarticulardisease, and use of systemic corticosteroid treatment.ThemortalityrateinJIAbasedonreportsfromthe United States and Canada is 0.29 per 100 patients,and most deaths occur in patients who have systemicJIA. (1)Summary• Juvenile idiopathic arthrithis (JIA) is the mostcommon rheumatic disease of childhood.• JIA is a chronic disease that is associated with periodsof disease flares and periods of disease inactivity.• Early, aggressive treatment with nonsteroidal antiinflammatorydrugs, intra-articular corticosteroidinjections, or methotrexate, has significantly improvedthe outcome of most children who have JIA.• Biologics have been shown to be both safe andeffective for the treatment of more aggressive formsof arthritis and for uveitis. Long-term safety data ofbiologics is still uncertain.• In the near future, it is hoped that genetic testing willallow earlier diagnosis of JIA as well as help predictthe disease course of children who have JIA. Geneticanalysis also may allow physicians to target therapiesmore effectively.• It is hoped that development of more specifictherapies will decrease overall immunosuppressionand other associated toxicities.References1. Weiss JE, Ilowite NT. Juvenile idiopathic arthritis. Rheum DisClin North Am. 2007;33(3):441–470, vi2. Ravelli A, Martini A. Juvenile idiopathic arthritis. Lancet. 2007;369(9563):767–7783. Rabinovich CE. Juvenile rheumatoid arthritis. Emedicine. Availableat: http://emedicine.medscape.com/article/1007276. AccessedOctober 6, 20<strong>11</strong>4. Woo P, Colbert RA. <strong>An</strong> overview of genetics of paediatric rheumaticdiseases. Best Pract Res Clin Rheumatol. 2009;23(5):589–5975. Prakken BJ, Albani S. Using biology of disease to understandand guide therapy of JIA. Best Pract Res Clin Rheumatol. 2009;23(5):599–6086. CassidyJT,PettyRE,LaxerRM,LindsleyCB.Textbook ofPediatric Rheumatology. 5th ed. Philadelphia, PA: Elsevier Inc.;20057. McCann LJ, Wedderburn LR, <strong>Has</strong>san N. Juvenile idiopathicarthritis. Arch Dis Child Educ Pract Ed. 2006;91:ep29–ep368. Quartier P. Current treatments for juvenile idiopathic arthritis.Joint Bone Spine. 2010;77(6):5<strong>11</strong>–5169. Foeldvari I, Nielsen S, Kümmerle-Deschner J, et al. Tumornecrosis factor-alpha blocker in treatment of juvenile idiopathicarthritis-associated uveitis refractory to second-line agents: results ofa multinational survey. J Rheumatol. 2007;34(5):<strong>11</strong>46–<strong>11</strong>50PIR QuizThis quiz is available online at http://www.pedsinreview.aappublications.org. NOTE: Since January 2012,learners can take Pediatrics in Review quizzes and claim credit online only. No paper answer form will be printedin the journal.New Minimum Performance Level RequirementsPer the 2010 revision of the American Medical Association (AMA) Physician’s Recognition Award (PRA) andcredit system, a minimum performance level must be established on enduring material and journal-based CMEactivities that are certified for AMA PRA Category 1 Credit TM . To successfully complete 2012 Pediatrics in Reviewarticles for AMA PRA Category 1 Credit TM , learners must demonstrate a minimum performance level of 60% orhigher on this assessment, which measures achievement of the educational purpose and/or objectives of thisactivity.Starting with 2012 Pediatrics in Review, AMA PRA Category 1 Credit TM can be claimed only if 60% or more of thequestions are answered correctly. If you score less than 60% on the assessment, you will be given additionalopportunities to answer questions until an overall 60% or greater score is achieved.312 Pediatrics in Review Vol.33 No.7 July 2012Downloaded from http://pedsinreview.aappublications.org/ by Enrique Mendoza-Lopez on July 2, 2012
collagen vascular disordersjuvenile idiopathic arthritis1. You are evaluating a 10-<strong>year</strong>-<strong>old</strong> girl for joint pain that has been present for w2 months. She has no fever butcomplains of pain and swelling in her hands and feet, which is worse in the morning. On physical examination,she has evidence of symmetric swelling of all proximal interphalangeal joints in her hands and feet and painover her temporomandibular joint. The remainder of the examination is normal. Which of the following is themost likely diagnosis?A. Enthesitis-related arthritisB. Oligoarticular juvenile idiopathic arthritis (JIA)C. Polyarticular JIAD. Psoriatic arthritisE. Systemic-onset JIA2. After determining the diagnosis in the patient mentioned above, you decide to initiate therapy for her arthritis.Which of the following medications is the most appropriate first medication to begin?A. CelecoxibB. InfliximabC. MethotrexateD. NaproxenE. Prednisone3. Which of the following statements regarding JIA is true?A. African American children more often have systemic-onset JIA than other subtypes.B. Association with HLA-B27 positivity is typical in enthesitis-related arthritis.C. Oligoarthritis occurs most commonly in adolescents.D. Polyarthritis occurs most commonly in male subjects.E. Psoriatic arthritis is not associated with ophthalmologic disease.4. Which of the following patients who have JIA is most likely to develop iritis?A. A 15-<strong>year</strong>-<strong>old</strong> boy who has enthesitis-related arthritis, antinuclear antibody (ANA)-negativeB. A 3-<strong>year</strong>-<strong>old</strong> girl who has oligoarticular subtype, ANA-negativeC. A 6-<strong>year</strong>-<strong>old</strong> girl who has oligoarticular subtype, ANA-positiveD. A 12-<strong>year</strong>-<strong>old</strong> girl who has polyarticular subtype, ANA-positiveE. A 5-<strong>year</strong>-<strong>old</strong> boy who has systemic onset subtype, ANA-negative5. A 7-<strong>year</strong>-<strong>old</strong> girl has developed a limp and complains of pain in her right knee, which is warm and swollen.Although she is afebrile in the office, her parents say she had a fever at home. You suspect oligoarticular JIAbut have concerns about infection. Which of the following tests would give you a definitive answer?A. ANAB. Erythrocyte sedimentation rateC. Rheumatoid factorD. Synovial fluid analysisE. White blood cell countDownloaded from http://pedsinreview.aappublications.org/ by Enrique Mendoza-Lopez on July 2, 2012Pediatrics in Review Vol.33 No.7 July 2012 313
ArticlerespiratorySudden Infant Death Syndrome: <strong>An</strong> UpdateRachel Y. Moon, MD,*Linda Fu, MD, MSc †Author DisclosureDrs Moon and Fu havedisclosed no financialrelationships relevantto this article. Thiscommentary does notcontain a discussion ofan unapproved/investigative use ofa commercial product/device.Educational Gap• Although the rate of sudden infant death syndrome (SIDs) deaths has remainedconstant–approximately 2,300 infants annually–since 2001, many deaths thatpreviously would have been classified as SIDS now are attributed to other sleep-relatedcauses.• The American Academy of Pediatrics Task Force on SIDS recently published a newPolicy Statement and Technical Report providing evidence-based guidance on theother causes of sleep-related infant deaths, such as soft bedding, prone sleep position,and bed sharing.Objectives After completing this article, readers should be able to:1. Discuss possible etiologic mechanisms for sudden infant death syndrome (SIDS).2. Identify the risk factors for SIDS.3. Discuss the American Academy of Pediatrics SIDS Task Force recommendations andunderlying rationale.4. Discuss the most common reasons for nonadherence with SIDS risk reductionrecommendations.IntroductionIn 2007, Pediatrics in Review published a review article on sudden infant death syndrome(SIDS). (1) This article uses that article as a reference and provides an update on thetopic.What We Knew ThenDefinitionSIDS is defined as a sudden unexplained death before 1 <strong>year</strong> of age. The death usually occursin a previously healthy infant, and the cause of death remains unexplained despitea thorough case investigation, including a complete autopsy, death scene investigation,and review of the clinical history.EpidemiologyIn the United States, w2,300 infants die of SIDS each <strong>year</strong>. Despite the success of the Backto Sleep campaign, which is associated with a steady declinein deaths from SIDS from the beginning of the campaign inAbbreviationsASSB: accidental suffocation and strangulation in bed1994 up to 2000, SIDS remains the third leading cause ofdeath in infancy and the most common cause of death between1 month and 1 <strong>year</strong> of age. Since 2001, the SIDS rateLB: live birthhas remained constant. In addition, the rate of otherOR: odds ratiosudden unexpected infant deaths, such as suffocation, asphyxia,SIDS: sudden infant death syndromeand other ill-defined or unspecified causes of death,*Director, Academic Development, Associate Chief, Division of General Pediatrics and Community Health, G<strong>old</strong>berg Center forCommunity Pediatric Health, Children’s National Medical Center; Professor of Pediatrics, George Washington University School ofMedicine and Health Sciences, Washington, DC.† Children’s National Medical Center; Assistant Professor, Pediatrics, George Washington University School of Medicine and HealthSciences, Washington, DC.314 Pediatrics in Review Vol.33 No.7 July 2012Downloaded from http://pedsinreview.aappublications.org/ by Enrique Mendoza-Lopez on July 2, 2012
espiratorysudden infant death syndromehas risen. Many deaths that previously would have beenclassified as SIDS are now being classified as having resultedfrom these other causes of death (see later in thisarticle).Risk factors for SIDS have been identified through epidemiologicstudies. SIDS is more likely to occur in maleinfants, at a 3:2 ratio. Other risk factors include proneand side sleeping position, maternal smoking during pregnancy,environmental tobacco smoke, overheating, softbedding, inadequate prenatal care, young maternal age,prematurity or low birth weight, and African Americanor American Indian/Alaska Native heritage.PathophysiologySIDS is a polygenic, multifactorial condition, with genetic,environmental, and behavioral/sociocultural factors ascontributors. Failure of arousal mechanisms likely playsan important role in the final pathway to death. Serotoninreceptor abnormalities have been found throughout theventral medulla in victims of SIDS, possibly representinga network dysfunction that affects arousal and cardiorespiratoryresponses. In addition, several studies have demonstratedpolymorphisms in the promoter region of aserotonin transporter protein gene known as 5-HTT, withalleles that increase promoter effectiveness (resulting inreduced serotonin concentrations at nerve endings),which are found more often in infants who have diedof SIDS.Polymorphisms also have been found in other genesthat may be pertinent in SIDS, such as SCN5A, a sodiumchannel gene that is related to a prolonged QT interval,and genes affecting autonomic nervous system development.Thus, it appears that certain infants may have a geneticpredisposition to SIDS, which becomes manifest when theinfant experiences some type of environmental challenge,such as prone positioning or tobacco exposure.What We Have Learned Since ThenEpidemiologyAs mentioned, there have been increases in other causesof sudden and unexpected infant death. Largely becauseof advances and improved training in death scene investigation,many deaths that previously would have been classifiedas SIDS are now being classified as the result of othercauses of sleep-related infant death. In the past 20 <strong>year</strong>s,much of the decline in SIDS rates may be explained by increasingrates of these other causes of death (Fig 1). In particular,the infant mortality rate from accidental suffocationand strangulation in bed (ASSB) has quadrupled in recent<strong>year</strong>s. ASSB and ill-defined or unspecified deaths are associatedparticularly with soft bedding, prone sleep position,and bed sharing.There continue to be racial and ethnic disparities, notonly for SIDS, but for ASSB and ill-defined or unspecifieddeaths. For all three conditions, non-Hispanic black infantsand American Indian/Alaska Native infants havemuch higher mortality rates than other infants of otherracial and ethnic groups. SIDS rates for non-Hispanicblack infants (99/100,000 live births [LBs]) and AmericanIndian/Alaska Native infants (<strong>11</strong>2/100,000 LBs)are twice as high as those of non-Hispanic white infants(55/100,000 LBs) and almost four times those of Asian/Pacific Islander and Hispanic infants. ASSB rates for non-Hispanic black infants (32.4/100,000 LBs) and AmericanIndian/Alaska Native infants (44.0/100,000 LBs) aremore than double those of non-Hispanic white infants(12.9/100,000 LBs). Rates of infant death from ill-definedor unspecified causes for non-Hispanic black infants (47.3/100,000 LBs) and American Indian/Alaska Native infants(64.9/100,000 LBs) are also more than double those ofnon-Hispanic white infants (21.1/100,000 LBs).Risk ReductionSIDS risk reduction strategies have focused on eliminatingrisk factors that are associated with SIDS in epidemiologicstudies:1. Back to sleep for every sleep.2. Use a firm sleep surface.3. Keep soft objects and loose bedding out of the crib.4. Avoid tobacco smoke exposure during pregnancy andafter birth.5. Room sharing without bed sharing is recommended.6. Consider offering a pacifier at nap time and bedtime.7. Avoid overheating.8. Do not use home cardiorespiratory monitors as a strategyto reduce the risk of SIDS.Figure 1. Proportion of sleep-related infant deaths in theUnited States, 1995 to 2005. Data source: Linked birth/infantdeath records 1995–2005 on CDC WONDER online database.US Department of Health and Human Services, Centers forDisease Control and Prevention, National Center for HealthStatistics, Division of Vital Statistics.Downloaded from http://pedsinreview.aappublications.org/ by Enrique Mendoza-Lopez on July 2, 2012Pediatrics in Review Vol.33 No.7 July 2012 315
espiratorysudden infant death syndromeRisk ReductionThe American Academy of Pediatrics (AAP) Task Forceon SIDS recently published an updated policy statement(2) and technical report. (3) The reader is referred tothese documents for additional details.SLEEP POSITION. The prone position places infants athigh risk for SIDS (odds ratio [OR] 2.3–13.1). The sideposition places infants at similarly high risk for SIDS, andindeed the population-attributable risk for the side sleepposition is higher than that for the prone position. Recentstudies have demonstrated the possible mechanisms bywhich the prone position places infants at higher risk forSIDS. There is evidence suggesting that prone sleeping resultsin altered autonomic control of the infant cardiovascularsystem during sleep, particularly at 2 to 3 months ofage, and may result in decreased cerebral oxygenation.These data may provide more impetus for parents andcaregivers to place infants in the supine position, becausemany are still reluctant to do so.One common reason for the reluctance is fear that theinfant will choke or aspirate while in the supine position;this concern is particularly true when the infant has beendiagnosed with gastroesophageal reflux. It may be helpfulto explain to parents that the infant’s airway protectivemechanisms (including the gag reflex, which is often misinterpretedas choking or aspiration) will prevent aspiration.Indeed, multiple studies in different countries havedemonstrated no increased incidence of aspiration sincethe change to supine sleeping. Even infants with gastroesophagealreflux should be placed supine for sleep.Parents and caregivers also will use nonsupine sleeppositions if they perceive that the infant is uncomfortableor does not sleep well while supine. Infants are less likely toarouse when they are in the prone position; however, frequentawakenings in an infant should not be interpreted asbeing abnormal or undesirable. On the contrary, sleepingfor sustained periods should be interpreted as being undesirable,because the ability to arouse from sleep is an importantprotective response.BED SHARING. The AAP recommends that infantsshare a room with their parents without bed sharing. Thisarrangement has been shown to be safer than both bed sharing(when the infant sleeps on the same surface as anotherperson) and solitary sleeping (when the infant sleeps in a separateroom from the parent), and decreases the risk of SIDSby w50%. In addition, adult beds are not designed for infantsafety, and many accidental infant deaths attributable tosuffocation, entrapment, asphyxia, and ill-defined or unspecifiedcause occur when the infant is bed sharing with anotherperson. Infants are at highest risk of SIDS or accidentaldeath while bed sharing during the first 3 months of lifeand if they are born prematurely or with low birth weight.In recent <strong>year</strong>s, there has been increased concern about bedsharing among public health officials because of rising ratesof infant deaths occurring during bed sharing.The AAP believes that currently there is insufficient evidenceto recommend any bed-sharing situation as safe,particularly because not all risks associated with bed sharing(such as parental fatigue) can be controlled. Specificbed-sharing situations are especially hazardous. These situationsinclude:• When the infant is
espiratorysudden infant death syndromeCRIB AND BEDDING ACCESSORIES. Blankets, pillows,and other soft bedding can increase the risk of SIDS andsuffocation. It is dangerous to place pillows, quilts, comforters,sheepskins, and other soft surfaces under, on top of, orclose to the infant. These practices increase SIDS risk up to21-f<strong>old</strong>, particularly when the infant is placed prone in thepresence of soft bedding. Use of soft bedding also has beenassociated with accidental suffocation deaths. Therefore,the AAP recommends that infants sleep on a firm surface,without any soft or loose bedding in the area. Infant sleepclothing can be used in place of blankets.Parents and caregivers frequently place blankets and pillowsunder, on, or close to the sleeping infant. The mostfrequently cited reasons for use of blankets and pillows arethe perceptions that these items will make the infant sleepmore comfortably (ie, the infant will fall asleep morequickly or will sleep for a more sustained period of time,or that the infant will not be c<strong>old</strong> while asleep) or will keepthe infant safe from injury or falls. Pillows in particular areused to create a barrier to prevent falls or to cushion injuryif the infant moves.A qualitative study found that, although mothers wereaware of the need for a firm sleep surface, they believedthat they were following that recommendation if theyplaced padding (blankets or a pillow) in between a mattressand sheet, as long as the sheet was pulled tautly over thepadding. (4)Crib bumper pads or similar products generally are usedbecause of the perception that they will protect the infantfrom injury (eg, limb entrapment between crib slats orhead injury from hitting railings) and for esthetic reasons;however, there have been recent concerns about infantdeaths from suffocation, entrapment, and strangulation associatedwith bumper pads. In addition, studies indicatethat bumper pad use prevents only minor injuries. A recentstudy of crib injuries concluded that the risk of suffocationor strangulation far outweighed the potential benefits ofpreventing minor injury with bumper pad use. (5)Parents frequently use wedges and positioning devicesthat are marketed as products that will decrease the risk ofSIDS or gastroesophageal reflex. There is no evidencesuggesting that these devices are effective against SIDS,suffocation, or gastroesophageal reflux; however, therehave been reports of deaths from suffocation and entrapmentassociated with these devices. These devices thereforeare not recommended.It is important for providers to counsel parents andcaregivers about the importance of eliminating soft beddingfrom the infant’s sleep area. Because of the commonmisperception that these products will keep their infantssafe, parents may unknowingly place their infants at higherrisk for SIDS and accidental death when they use productssuch as blankets, pillows, bumper pads, and positioners.BREASTFEEDING. Recent reports, including a metaanalysis,(6) demonstrate a protective effect of breastfeeding(nursing or pumped human milk) against SIDS, with anapproximate halving of the risk when the baby is breastfed;the protective effect is even stronger when the infant isexclusively breastfed. Possible reasons for this protectiveeffect include decreased infectious diseases (which areassociated with increased risk of SIDS) and overall immunebenefits. In addition, physiologic studies demonstrate thatbreastfed infants are more easily aroused from sleep thanformula-fed infants.Infants can be brought into the adult bed for feeding;however, after feeding, when the mother is ready to goback to sleep, the infant should be returned to his orher own sleep area (ie, crib or bassinet). In addition, becauseof the extremely high risk of SIDS associated withsofas and armchairs, mothers who are likely to fall asleepwhile nursing should not nurse on a sofa or armchair.PACIFIERS. Multiple studies, including two metaanalyses,have found pacifierusetobeassociatedwithadecreased risk of SIDS (adjusted OR 0.39–0.48). (7)(8)The mechanism of action is unknown, but it is theorizedthat pacifier use may alter arousal thresh<strong>old</strong>s or autonomicresponses during sleep; however, the decreased risk ofSIDS persists even when (as frequently occurs) the pacifierfalls out of the infant’s mouth after falling asleep. A recentstudy has demonstrated that pacifier use may positivelymodify the effect of adverse risk factors, such as prone positioning.(9) The AAP recommends that pacifier use beencouraged as a SIDS risk reduction strategy.Many parents are concerned about the possible negativeeffect of pacifier use on breastfeeding success. Randomizedclinical trials have not shown pacifier use toresult in shortened breastfeeding duration, if the pacifierhas been introduced after 2 to 4 weeks of age. Pacifierscan be used for breastfed infants, but they should not beintroduced until breastfeeding has been well established.ROOM VENTILATION AND FANS. One US study foundthat the use of a fan decreased the risk of SIDS (adjustedOR 0.28; 95% confidence interval, 0.10–0.77). (10)This study, however, was limited by having a small sampleof fan users and by questions about the accuracy ofparental recall. Furthermore, although one other studyhas demonstrated a decreased risk of SIDS if the roomis well ventilated, no other studies have confirmed thesefindings. Therefore, there is currently no recommendationfor or against fan use as a SIDS risk reduction strategy.Downloaded from http://pedsinreview.aappublications.org/ by Enrique Mendoza-Lopez on July 2, 2012Pediatrics in Review Vol.33 No.7 July 2012 317
espiratorysudden infant death syndrome• It is recommended that blankets, pillows, and othersoft bedding be removed from the infant sleep area.(26)(27)• Overheating should be avoided. (<strong>11</strong>)(28)(29)(30)• Breastfeeding should be encouraged for SIDS riskreduction. (6)• Pacifier use should be encouraged for SIDS riskreduction. (7)(8)• Immunizations should be encouraged for SIDS riskreduction. (13)• The evidence for fan use or swaddling as strategies toreduce the risk of SIDS is inconclusive.References1. Moon RY, Fu LY. Sudden infant death syndrome. Pediatr Rev.2007;28(6):209–2142. Moon RY; Task Force on Sudden Infant Death Syndrome.Policy statement: SIDS and other sleep-related infant deaths:expansion of recommendations for a safe infant sleeping environment.Pediatrics. 20<strong>11</strong>;128(5):1030–10393. Moon RY; Task Force on Sudden Infant Death Syndrome.Technical report: SIDS and other sleep-related infant deaths:expansion of recommendations for a safe infant sleeping environment.Pediatrics. 20<strong>11</strong>;128(5):e1341–e13674. Ajao TI, Oden RP, Joyner BL, Moon RY. Decisions of blackparents about infant bedding and sleep surfaces: a qualitative study.Pediatrics. 20<strong>11</strong>;128(3):494–5025. Yeh ES, Rochette LM, McKenzie LB, Smith GA. Injuriesassociated with cribs, playpens, and bassinets among young childrenin the US, 1990-2008. Pediatrics. 20<strong>11</strong>;127(3):479–4866. Hauck FR, Thompson JM, Tanabe KO, Moon RY, VennemannMM. Breastfeeding and reduced risk of sudden infant deathsyndrome: a meta-analysis. Pediatrics. 20<strong>11</strong>;128(1):103–<strong>11</strong>07. Hauck FR, Omojokun OO, Siadaty MS. Do pacifiers reduce therisk of sudden infant death syndrome? A meta-analysis. Pediatrics.2005;<strong>11</strong>6(5):e716–e7238. Mitchell EA, Blair PS, L’Hoir MP. Should pacifiers be recommendedto prevent sudden infant death syndrome? Pediatrics.2006;<strong>11</strong>7(5):1755–17589. Moon RY, Yang DC, Tanabe KO, Young HA, Hauck FR.Pacifier use and SIDS: evidence for a consistently reduced risk.Matern Child Health J. 2012;16(3):609–61410. Coleman-Phox K, Odouli R, Li DK. Use of a fan during sleepand the risk of sudden infant death syndrome. Arch Pediatr AdolescMed. 2008;162(10):963–968<strong>11</strong>. Ponsonby A-L, Dwyer T, Gibbons LE, Cochrane JA, Wang YG.Factors potentiating the risk of sudden infant death syndrome associatedwith the prone position. NEnglJMed. 1993;329(6):377–38212. Blair PS, Sidebotham P, Evason-Coombe C, Edmonds M,Heckstall-Smith EM, Fleming P. Hazardous cosleeping environmentsand risk factors amenable to change: case-control study ofSIDS in south west England. BMJ. 2009;339:b366613. Vennemann MM, Höffgen M, Bajanowski T, Hense HW,Mitchell EA. Do immunisations reduce the risk for SIDS? A metaanalysis.Vaccine. 2007;25(26):4875–487914. Joyner BL, Gill-Bailey C, Moon RY. Infant sleep environmentsdepicted in magazines targeted to women of childbearing age.Pediatrics. 2009;124(3):e416–e42215. Kattwinkel J, Hauck FR, Keenan ME, et al; American Academyof Pediatrics Task Force on Sudden Infant Death Syndrome. Thechanging concept of sudden infant death syndrome: diagnosticcoding shifts, controversies regarding the sleeping environment,and new variables to consider in reducing risk. Pediatrics. 2005;<strong>11</strong>6(5):1245–125516. MacDorman MF, Cnattingius S, Hoffman HJ, Kramer MS,Haglund B. Sudden infant death syndrome and smoking in theUnited States and Sweden. Am J Epidemiol. 1997;146(3):249–25717. Schoendorf KC, Kiely JL. Relationship of sudden infant deathsyndrome to maternal smoking during and after pregnancy.Pediatrics. 1992;90(6):905–90818. Malloy MH, Kleinman JC, Land GH, Schramm WF. Theassociation of maternal smoking with age and cause of infant death.Am J Epidemiol. 1988;128(1):46–5519. Haglund B, Cnattingius S. Cigarette smoking as a risk factorfor sudden infant death syndrome: a population-based study. Am JPublic Health. 1990;80(1):29–3220. Willinger M, James LS, Catz C. Defining the sudden infantdeath syndrome (SIDS): deliberations of an expert panel convenedby the National Institute of Child Health and Human Development.Pediatr Pathol. 1991;<strong>11</strong>(5):677–68421. Committee on Environmental Health; Committee on SubstanceAbuse; Committee on Adolescence; Committee on NativeAmerican Child. From the American Academy of Pediatrics: Policystatement—Tobacco use: a pediatric disease. Pediatrics. 2009;124(5):1474–148722. Best D; Committee on Environmental Health; Committee onNative American Child Health; Committee on Adolescence. Fromthe American Academy of Pediatrics: Technical report—Secondhandand prenatal tobacco smoke exposure. Pediatrics. 2009;124(5):e1017–e104423. Tappin D, Ecob R, Brooke H. Bedsharing, roomsharing, andsudden infant death syndrome in Scotland: a case-control study.J Pediatr. 2005;147(1):32–3724. Blair PS, Fleming PJ, Smith IJ, et al. Babies sleeping withparents: case-control study of factors influencing the risk of thesudden infant death syndrome. CESDI SUDI research group. BMJ.1999;319(7223):1457–146125. Carpenter RG, Irgens LM, Blair PS, et al. Sudden unexplainedinfant death in 20 regions in Europe: case control study. Lancet.2004;363(9404):185–19126. Hauck FR, Herman SM, Donovan M, et al. Sleep environmentand the risk of sudden infant death syndrome in an urbanpopulation: the Chicago Infant Mortality Study. Pediatrics. 2003;<strong>11</strong>1(5 part 2):1207–121427. Kemp JS, Unger B, Wilkins D, et al. Unsafe sleep practices andan analysis of bedsharing among infants dying suddenly andunexpectedly: results of a four-<strong>year</strong>, population-based, death-sceneinvestigation study of sudden infant death syndrome and relateddeaths. Pediatrics. 2000;106(3):E4128. Fleming PJ, Gilbert R, Azaz Y, et al. Interaction betweenbedding and sleeping position in the sudden infant death syndrome:a population based case-control study. BMJ. 1990;301(6743):85–8929. Ponsonby A-L, Dwyer T, Gibbons LE, Cochrane JA, JonesME, McCall MJ. Thermal environment and sudden infant deathsyndrome: case-control study. BMJ. 1992;304(6822):277–28230. Iyasu S, Randall LL, Welty TK, et al. Risk factors for suddeninfant death syndrome among northern plains Indians. JAMA.2002;288(21):2717–2723Downloaded from http://pedsinreview.aappublications.org/ by Enrique Mendoza-Lopez on July 2, 2012Pediatrics in Review Vol.33 No.7 July 2012 319
espiratorysudden infant death syndromePIR QuizThis quiz is available online at http://www.pedsinreview.aappublications.org. Note: Since January 2012, learners can take Pediatricsin Review quizzes and claim credit online only. No paper answer form will be printed in the journal.New Minimum Performance Level RequirementsPer the 2010 revision of the American Medical Association (AMA) Physician’s Recognition Award (PRA) and credit system,a minimum performance level must be established on enduring material and journal-based CME activities that are certified for AMAPRA Category 1 Credit TM . To successfully complete 2012 Pediatrics in Review articles for AMA PRA Category 1 Credit TM , learnersmust demonstrate a minimum performance level of 60% or higher on this assessment, which measures achievement of theeducational purpose and/or objectives of this activity.Starting with 2012 Pediatrics in Review, AMA PRA Category 1 Credit TM can be claimed only if 60% or more of the questions areanswered correctly. If you score less than 60% on the assessment, you will be given additional opportunities to answer questionsuntil an overall 60% or greater score is achieved.1. Of the following, an epidemiologic risk factor associated with sudden infant death syndrome (SIDS) is:A. Caucasian raceB. Female genderC. Paternal alcohol useD. Post-term gestationE. Prone sleeping position2. Abnormalities in the receptor for which of the following neuropeptides have been identified in the brainstemof some infants with sudden infant death syndrome:A. AcetylcholineB. EpinephrineC. GalaninD. SerotoninE. Vasoactive intestinal peptide3. <strong>An</strong> intervention recommended by the authors to reduce the risk of infant SIDS is:A. Avoiding the use of pacifiers before bedtimeB. Home cardiorespiratory monitoringC. Positioning the infant on the sideD. Sleeping in the same room as the infantE. Swaddling the infant in a soft blanket4. The American Academy of Pediatrics Task Force on SIDS has recommended against parents sleeping in the same bedas their infants. According to the article, which of the following factors makes bed sharing especially hazardous?A. Absence of blanketsB. Firm bedC. Infant age
question from the clinicianRisk of ThrombophiliaLeonardo R. Brandao, MD, MSc,* George B. Segel, MD †Author DisclosureDrs Brandao and Segel have disclosedno financial relationships relevant tothis article. This commentary does notcontain a discussion of anunapproved/investigative use ofa commercial product/device.QuestionA 17-<strong>year</strong>-<strong>old</strong> girl meets with you todiscuss her use of oral contraceptives(OCPs) that contain estrogen. In thefamily history focusing on evidence ofthrombophilia, you find that the patient’sfather died of a pulmonary embolus afterdisembarking from a transatlantic airplaneflight. The patient and her motherwant to know if she should use OCPs.The girl is leaving for college, andthere is concern for an unwanted pregnancyon the one hand and the risk ofthrombosis on the other. How shouldyou advise her?<strong>An</strong>swerIn this case, the family history providesa vital piece of information inassessing risk of thrombosis for thispatient using OCPs. The currentguidelines of the American Collegeof Obstetrics and Gynecology donot recommend laboratory testingfor thrombophilia if a careful familyhistory is unrevealing. (1) In view ofthe father’s pulmonary embolus anddemise, a consideration of inheritedabnormalities that may predispose toa thrombus and acquired conditionsthat may provoke a thrombus is warranted.The father’s long airplane ride,particularly if it was in the coach sectionwith limited mobility, is a risk factorfor a lower extremity or pelvicthrombus and consequent pulmonaryembolus, although at least one-thirdto one-half of the adult patients whoexperience pulmonary emboli do not*Assistant Professor of Paediatrics, Division ofHaematology/Oncology, University of Toronto, TheHospital for Sick Children, Toronto, Ontario.† Professor of Medicine, Professor Emeritus ofPediatrics, University of Rochester School ofMedicine, Rochester, NY.have an identifiable source of the embolization.(2) The thrombotic riskwould be heightened if he had an underlyingmalignancy or inflammatorydisease, but there was no history of eitherof these conditions.If the immobilization of the airplaneride were coupled with an inheritedthrombophilic condition, suchas factor V Leiden (5% of the population),the risk would be more than additive.In the scenario presented, wehave no information about an underlyingthrombophilic mutation; but thepopulation frequency of such abnormalitiesmakes it reasonable to explorethis possibility in this young woman inview of her positive family history,because such a mutation would increaseher risk of a thrombus if she usedOCPs.<strong>An</strong> established thrombophilia panelincludes genetic and plasma-basedtesting, (3)(4)(5) as follows:• Inherited genetic factors: (1) FactorV Leiden, a single point mutationin coagulation factor Vpresent in 5% of whites, renderingit resistant to inhibition by activatedprotein C. (2) ProthrombinG20210A mutation, a second inheritedthrombophilic conditionthat occurs in w2% of the population.This mutation represents anabnormally regulated gene forthe production of coagulation factorII that may result in high,prothrombotic levels of prothrombin.(3) Diminished amounts of naturalanticoagulants: proteins C, S, orantithrombin.• Acquired prothrombotic factors:anticardiolipin antibody, lupus anticoagulantantibody, and heightenedfactor VIII levels. Althoughseen in inflammatory states, thesePediatrics in Review Vol.33 No.7 July 2012 321
question from the clinicianfactors may occur in the absence ofan underlying condition.• Other elements of a comprehensivethrombophilia panel include considerationof high fasting plasmalevels of lipoprotein (a) or homocysteine,and high circulating levelsof factors IX and XI, but theimpact of these factors on the developmentof thromboembolism isnot certain.The results of plasma-derived coagulationtests performed in childrenshould be compared with their respectiveage-appropriate values. (6) Furthermore,each thrombophilic factornoted presents a different risk for thedevelopment of a thrombus. (7)The routine testing of children forboth acquired and inherited conditionsis controversial, given the lowprevalence of thrombotic events, theeven lower prevalence of unprovokedevents, and the variable presence of externalrisk factors (eg, limited mobility,central lines, estrogen-containing OCPmedications). Thrombophilia testing todetermine the duration of anticoagulationin children or to prevent venousthromboembolism or life-threateningevents in adolescent patients beingconsidered for oral contraception hasnot been recommended in the absenceof a family or patient history of thrombosis.(8)(9)The patient in the case describedshould have a complete evaluationfor a thrombophilic factor. If a riskfactor is identified, estrogen-containingOCPs should not be used. If an abnormalityis detected, it would bebeneficial to take a multidisciplinaryapproach, involving a pediatric hematologistand a gynecologist, to considerthe different contraceptive methodsthat are compatible with the resultsof the thrombophilia investigation.(10) Current data do not indicateany heightened risk of thrombosis withthe use of progesterone alone as a contraceptive,and numerous barrier methodsof contraception are available.Importantly, additional modifiable riskfactors such as obesity, inappropriatediet and sedentarism, and exposureto alcohol and tobacco also need tobe addressed.References1. ACOG Practice Bulletin No 73. Clinicalmanagement guidelines for obstetriciangynecologists.Obstet Gynecol. 2006;107(6):1453–14722. Streiff MB, Bockenstedt PL, CatalandSR, et al. Venous thromboembolic disease.J Natl Compr Canc Netw. 20<strong>11</strong>;9(7):714–7773. Segel GB, Francis CA. <strong>An</strong>ticoagulantproteins in childhood venous and arterialthrombosis: a review. Blood Cells Mol Dis.2000;26(5):540–5604. Ruiz-Irastorza G, Crowther M, BranchW, Khamashta MA. <strong>An</strong>tiphospholipid syndrome.Lancet. 2010;376(9751):1498–15095. Crowther MA, Kelton JG. Congenitalthrombophilic states associated with venousthrombosis: a qualitative overview and proposedclassification system. <strong>An</strong>n InternMed. 2003;138(2):128–1346. Monagle P, Barnes C, Ignjatovic V, et al.Developmental haemostasis. Impact forclinical haemostasis laboratories. ThrombHaemost. 2006;95(2):362–3727. Young G, Albisetti M, Bonduel M, et al.Impact of inherited thrombophilia on venousthromboembolism in children: a systematicreview and meta-analysis of observationalstudies. Circulation. 2008;<strong>11</strong>8(13):1373–13828. O’Brien SH, Smith KJ. Using thrombophiliatesting to determine anticoagulationduration in pediatric thrombosis is notcost-effective. J Pediatr. 2009;155(1):100–1049. Gomes MP, Deitcher SR. Risk of venousthromboembolic disease associatedwith hormonal contraceptives and hormonereplacement therapy: a clinical review.Arch Intern Med. 2004;164(18):1965–197610. Trenor CC III, Chung RJ, MichelsonAD, et al. Hormonal contraception andthrombotic risk: a multidisciplinary approach.Pediatrics. 20<strong>11</strong>;127(2):347–357322 Pediatrics in Review Vol.33 No.7 July 2012
ethics for the pediatricianThe Evolving Ethics of CochlearImplants in ChildrenJohn D. Lantos, MDAuthor DisclosureDr Lantos has disclosed no financialrelationships relevant to this article.This commentary does not containa discussion of an unapproved/investigative use of a commercialproduct/device.AbbreviationsFDA: Food and DrugAdministrationNAD: National Association of theDeafNIH: National Institutes of HealthIntroductionA cochlear implant is a small, complexelectronic device that can help to providea sense of sound to a person whois profoundly deaf or severely hardof-hearing.Implants all have fourkey components: (1) a microphone,which picks up sound from the environment;(2) a processor, which selectsand arranges sounds picked upby the microphone; (3) a transmitterand receiver/stimulator, which convertssignals from the processor intoelectric impulses; and (4) a group ofelectrodes that sends impulses fromthe stimulator to different regions ofthe auditory nerve. After implantationof the device, recipients must undergoan extensive program of speech therapytolearnhowtomaketheimplantuseful. As of December 2010,w219,000 people had received implantsworldwide, including 71,000people in the United States, of whom28,400 were children. (1)Over the past two decades, doctors,parents, policymakers, and membersof the Deaf community havestruggled to understand the benefits,risks, and implications of using cochlearimplants for children. Thesestruggles were the latest episode in adebate about medical and educationalapproaches to deafness that have lastedover a century. They also reflected thefact that there were no good long-termoutcome data on cochlear implants.Thus, complex issues of clinical and researchethics are intertwined with complexcultural issues and a long historyof discrimination and stigmatizationUniversity of Missouri at Kansas City; Children’sMercy Bioethics Center, Children’s Mercy Hospital,Kansas City, MO.of children who are deaf. In this article,I will review the origins of the controversy,discuss its most heated moments,and summarize the current state of thedebate.Origins of the ControversyMuch of the ethical controversy aboutcochlear implants reflects a debatethat has been going on for >100 <strong>year</strong>sabout the best way to educate childrenwho are deaf in order to maximizetheir opportunities in life. Some people,notably Thomas Gallaudet, wereof the opinion that children who aredeaf all should be taught sign languageand educated by teachers who knowsign language. This approach becameknown as “manualism.” Others, notablyAlexander Graham Bell and HoraceMann, thought it was preferable to educatechildren who are deaf to speakand to read lips. This philosophy wasknown as “oralism.”Bell, whose wife was deaf, was himselffluent in sign language. He arguedthat sign language should be discouragedbecause it would lead to isolationof individuals who are deaf from hearingpeople. He also feared that moremarriages between people who aredeaf would result in a perpetuationof deafness. Thus, his program wasmotivated by eugenic concerns as wellas educational considerations. Bell wentas far as advocating a ban on marriagesbetween people who are deaf. (2)Although Bell’s extreme viewsabout marriage never prevailed, hisviews on the superiority of oralismover signing did prevail in many schoolsfor the deaf. Throughout the early 20thcentury, many such schools prohibitedsigning. (3) By the middle of the 20thcentury, however, oralism had beenDownloaded from http://pedsinreview.aappublications.org/ by Enrique Mendoza-Lopez on July 2, 2012Pediatrics in Review Vol.33 No.7 July 2012 323
ethics for the pediatriciandiscredited thoroughly. A 1964 reportto Congress on deaf education calledoralism a “dismal failure.” The reportrecommended new approaches to theeducation of children who are deaf.(4) Oralism failed, in part, becauselip reading (or “speechreading”) isdifficult and never perfect. Onlyw30% to 35% of English sounds canbe speechread. Words as different as“queen” and “white” look the sameon a person’s lips. So do the threewords in the sentence, “Buymypie”.(5) Sign language, by contrast, allowsstudents who are deaf to understandall that is being communicated.In recent <strong>year</strong>s, this stark exclusivityhas all but disappeared. Most educatorstoday prefer methods thatcombine lip reading, speech, and signlanguage, and are known collectivelyas “total communication”. (6) Still, the<strong>year</strong>s during which signing was prohibitedin schools for the deaf left deepscars. Signing became a matter of deeppride and cultural identity within theDeaf community. (7) This history explains,in part, the response of many inthe Deaf community to cochlear implants.They saw implants as a returnto the philosophy of oralism and a rejectionof sign language.The Controversy OverCochlear Implants forChildrenIn 1957, Djourno and Eyries were thefirst surgeons to implant an electrodein a patient’s cochlea. (8) Over the nextdecades, others developed the technology.Between 1965 and 1970, Housedeveloped single-electrode cochlearimplants and teamed up with 3M tomake the first commercial cochlearimplants in the United States. Thesedevices were first marketed in 1972,and in 1984 they were approved bythe Food and Drug Administration(FDA) for use in adults.In the mid-1970s, Clarke developeda multielectrode cochlear implant, andfirst implanted an adult patient withhis prototype device in 1978. Multichanneldevices divide the incomingsignal into various frequency bandsthat are then transmitted to varioussites of stimulation spanning the innerear. Low-pitch sounds are sent to onepart of the cochlea, high-pitch soundsto another, more closely mimickingthe human ear. Because multiplechannels provide a more detailedrepresentation of sound, they arethought to allow better speech understandingthan do single-channeldevices. Clarke’s multichannel devicewas approved in 1985. Throughoutthese <strong>year</strong>s, implants were being offeredto children, although they werenot yet approved by the FDA for usein pediatric populations.In 1990, the FDA approved theuse of cochlear implants for childrenover age 2 <strong>year</strong>s. This decision led toa firestorm of controversy. The next<strong>year</strong>, the National Association of theDeaf (NAD) issued a statement deploringthe FDA’s approval decision.(9) They argued that cochlear implantsfor children remained highlyexperimental. They accused the FDAof “failing to consult formally withorganizations of deaf Americans andwith deaf leaders and scholars knowledgeableabout the acquisition anduse of sign communication and Englishin deaf children, the psychosocialdevelopment and educationof deaf children, and the social organizationand culture of the AmericanDeaf community.” Most controversially,the NAD claimed that deafness“comprises a linguistic and culturalminority,” and that “we should notseek the scientific tools nor use them,if available, to change a child biologicallyso he or she will belong to themajority rather than the minority –even if we believe that this biologicalengineering might reduce the burdensthe child will bear as a memberof a minority.” They recommendeda national conference to address theethical issues surrounding cochlearimplants for children.In 1995, a conference was convenedat the National Institutes ofHealth (NIH) to address some ofthe issues raised by cochlear implants.Of note, it was not the conferencethat the NAD had recommended.Instead, the scope was much morelimited. According to the organizers,“The conference was convened tosummarize current knowledge aboutthe range of benefits and limitationsof cochlear implantation that have accruedto date. Such knowledge is animportant basis for informed choicesfor individuals and their familieswhose philosophy of communicationis dedicated to spoken discourse. Issuesrelated to the acquisition of signlanguage were not addressed directlyby the panel, because the focus ofthe conference was on new informationon cochlear implant technologyand its use. The panel acknowledgesthe value and contributions of bilingualand bicultural approaches todeafness”. (10) Conference panelistswere drawn from “the fields ofotolaryngology, audiology, speechlanguagepathology, pediatrics, psychology,and education, and includinga public representative.” There was norepresentative from Deaf culture. Thegoal of the conference was to makerecommendations to the NIH andthe FDA about cochlear implants.More than 600 people attended.The conclusions of the consensusconference were as follows: “Cochlearimplantation improves communicationability in most adults with severeto-profounddeafness and frequentlyleads to positive psychological andsocial benefits as well. Currently,children at least 2 <strong>year</strong>s <strong>old</strong> and adultswith profound deafness are candidatesfor implantation. Cochlear implantcandidacy should be extended to adultswith severe hearing impairment and324 Pediatrics in Review Vol.33 No.7 July 2012Downloaded from http://pedsinreview.aappublications.org/ by Enrique Mendoza-Lopez on July 2, 2012
ethics for the pediatricianopen-set sentence discrimination thatis less than or equal to 30% in thebest-aided condition. Access to optimaleducation and (re)habilitationservices is important for adults andis critical for children to maximizethe benefits available from cochlearimplantation.”Critics of the NIH conclusionspointed out that there were no rigorous,controlled studies of outcomesfor children. In particular, they worriedthat only surrogate end pointsand controversial markers of languageacquisition were being used. In a reviewpublished in 1997, Lane andGrodin noted that “the decision tohave the surgery and habilitationmay promote delays in the child’sacquisition of ASL [American SignLanguage] and therefore may delaythe time when that child has any fulllanguage at his or her command. Developmentalmilestones for signedlanguages are similar to those forspoken languages, and the later theacquisition of ASL, the poorer its masteryon the average. If implanted childrenbecome fluent neither in Englishnor in ASL, their intellectual, social,and psychological development maybe compromised”. (<strong>11</strong>)In a comprehensive review publishedin 1998, Lane and Bahan notedthat, until long-term outcome studieswere done to quantify linguistic, psychosocial,and educational attainmentsof implanted children, theprocedure should be considered innovativeor experimental and should beconducted only in carefully designedresearch protocols. (12) These articleswere asking questions that were partlymethodological, partly ethical. By askingwhich outcome measures shouldbe used, they were asking how to definesuccess. Should it be word recognition?School performance? Qualityof life? Or something else?At this point, both sides in the battleseemed to have firm and incompatiblepositions. On the one hand, the surgeonsand audiologists were enamoredof the new technology andeven, perhaps, a little starry-eyed aboutthe potential benefits. Reports in themid-1990s often included individualcase reports, but no careful studiesof series of patients who had receivedimplants. (13) Such individualcase reports were usually optimistic,but not scientifically rigorous. Thus,members of the Deaf communityremained skeptical and unsure ofthe efficacy or the implicationsof this new technology. These criticswithin the Deaf communityseemed to be the voice of reason,calling for careful study and evaluation.But the real-world situationwas never really so simple ordichotomous.Some advocates within the Deafcommunity, as well as some bioethicists,suggested that even a treatmentthat would be 100% effective (whatis 100% effective?) was undesirable.(14) Some of these critics saw attemptsto treat or cure deafness asa genocidal attack on the Deaf community,an extension of the oralisttradition of Bell and others. <strong>An</strong> articlein The Washington Post in 1997described this group as “a radicalsegment” who “.call cochlear implants‘the devil’s work;’ who considerMiss America 1995, the deafHeather Whitestone, a charlatan becauseshe speaks; who picket oralistdeaf schools and stop parents of childrenwith implants in malls, demandingto know why they butcher deafbabies”. (15) In the eyes of this group,cochlear implants were seen as an interventionthat was offered not toimprove the outcome for individualchildren but, instead, as an assaulton the Deaf community. Given theseviews, it seemed that there could beno reconciliation in views betweentheproponentsofimplantsandtheiropponents.A Mysterious AttitudeShiftThen, somehow, and rather suddenly,the terms of the controversy seemedto change. The Deaf community softenedits opposition. Cochlear implantscame to be seen as one acceptable optionamong many. The reasons for thetransition in attitudes are complex.One explanation for the shift wasthat it was a response to public pressureon the NAD. This is the view ofI. King Jordan, President Emeritus ofGallaudet University: “The NAD positionreceived adverse attention fromthe media and criticism from moderatesin the deaf community and fromphysicians and allied health professionalswho considered cochlear implantsan appropriate option”. (16)In response to this adverse attention,the NAD withdrew its controversialposition paper. In 2000, they releaseda new position paper that recognizescochlear implants as one of multipleoptions. Note that this change in outlookdoes not mean that the NAD’searlier concerns were wrong. Thenew statement endorsed informedparental choice, recognized that thereis diversity within the Deaf community,and supported parents’ right tomake the decision that they think bestfor their child.The NAD knows that parents love andcare deeply about their deaf children.Since the decision to perform implantsurgery on the deaf child is made forthe child, it is necessary for parents tobecome educated about cochlear implants– the potential benefits, the risks,and all the issues that they entail. Duringthis critical education process, parentshave both the need and the right to receiveunbiased information about thepros and cons of cochlear implants andrelated matters. The NAD knows thatparents want to make informed decisions.Parents also would benefit byopportunitiesto interact with successfuldeaf and hard of hearing adults, as wellas with parents of deaf and hard of hearingchildren. (17)Downloaded from http://pedsinreview.aappublications.org/ by Enrique Mendoza-Lopez on July 2, 2012Pediatrics in Review Vol.33 No.7 July 2012 325
ethics for the pediatrician<strong>An</strong>other reason why the debateshifted is that follow-up studies haveanswered some of the questions thatcould not be answered in the earlydays. When cochlear implants firstcame into use for children, it was notclear whether the implants would causemore harm than good by promisingmore hearing, and thus more verballinguistic functioning, than they coulddeliver. The earliest outcome studiesconfirmed some of these fears. Manychildren who received cochlear implantscontinued to have poor languageacquisition and to do poorly in school.Later studies showed that outcomeswere, in fact, variable. Some childrenwho receive implants are able to functionwell in the hearing world. Moreoften, however, they continue to requirespecial assistance or to use signlanguage along with spoken language.Thus, many children who have hada cochlear implant still go to schoolin classrooms for the deaf, or inhearing classrooms but with specialservices. For many such children,the approach has changed from aneither/or, oralism/manualism choiceto an approach that stresses bilingualism(that is, the use of both ASL andoral communication) in an approachthat is individualized and uses all availabletechnology. (18)Issues for the FutureSome issues remain contentious. Thequestion of whether parents who aredeaf should be able to choose to havea child who is deaf, either throughpreimplantation genetic diagnosis orthrough prenatal testing and terminationof pregnancy if the child isnot deaf, remains as contentious asever. (19) Some advocates for the deaf(20) and some bioethicists (21) insistthat this option should be available toparents who want an infant who is deaf.Others insist that it is unethical, eventhough understandable, for parents tochoose deafness for a child. (22)Of course, some forms of reproductionare easier to regulate thanothers. Couples may be denied accessto preimplantation genetic diagnosis.But it is difficult to imagine regulationsthat would prevent two carriersof an autosomal dominant form ofdeafness from having children together.Questions have arisen about thecost of cochlear implants and of accessto both the technology and theextensive postsurgical habilitation thatis necessary. A recent study fromCleveland showed that poor childrenwere just as likely to receive a cochlearimplant as children of higher socioeconomicstatus, but that they had morecomplications and poorer followupcare. (23) Such outcomes arenot unique to cochlear implants butare true for many medical treatmentsthat require long-term follow-up. Althoughdisparities in access to healthcare are common throughout medicineand throughout the world, the issuessurrounding deafness raise uniqueissues, because most newborns in theUnited States are now screened forhearing loss. (24) In general, screeningprograms should be implemented onlyif there is access to appropriate treatmentfor those who test positive. (25)Summary• The story of the ethicalcontroversy over cochlearimplants is unique in some waysand paradigmatic in others. It isunique in the ways that it wasshaped by the history of deafness,and of cultural responses todeafness, in the United States.• The story is paradigmatic in twoways. First, cochlear implantationwas an innovative therapy that wasintroduced into practice withoutadequate study. Promising earlytrials led to FDA approval, althoughlong-term outcome data fromrigorous studies were lacking. Inthis respect, the story of cochlearimplants is similar to the history ofother innovations that wereintroduced without rigorousevaluation, innovations such assupplemental oxygen, (26)extracorporeal membraneoxygenation, (27) orcorticosteroids forbronchopulmonary dysplasia. (28)• Cochlear implants also areparadigmatic of a particular typeof ethical dilemma in whichadvocacy groups claim to knowbetter what is best for childrenthan do the children’s parents ordoctors. This controversy happenedduring the Baby Doe debate in the1980s, when advocacy groupsclaimed that doctors and parentswere conspiring to discriminateagainst children with disabilities.(29) Ultimately, the US SupremeCourt invalidated thatinterpretation of disability rights.(30) Instead, parents and doctorsworking together are givendiscretion to make decisionsaboutwhatisbestforchildren.• With regard to cochlear implantsfor children, the NAD realizesthat they are walking a fine line.As one NAD spokesperson said,“We don’t say that hearingparents aren’t qualified to makedecisions about their deafchildren. We say that they needto have contact with deaf peopleif they’re going to make educateddecisions”. (7) The same could besaid for pediatricians.• There are 4,000 to 8,000 infantsborn each <strong>year</strong> in the United Stateswith severe hearing impairment.Their parents will have to makedecisions about what is best.• Pediatricians need to understandthe options and be prepared tohelp parents sort through thecomplexdataandmultipleoptionsin order to arrive at the decisionthat is best for themselves andtheir child. Understanding theethical controversy over cochlearimplants will help.To view the references for thisarticle, visit the July issue at http://pedsinreview.aappublications.org andclick on “Ethics for the Pediatrician.”326 Pediatrics in Review Vol.33 No.7 July 2012Downloaded from http://pedsinreview.aappublications.org/ by Enrique Mendoza-Lopez on July 2, 2012
index of suspicionCase 1: Limp in a 23-month-<strong>old</strong> <strong>Girl</strong>Case 2: Abnormal Eyelashes in 17-<strong>year</strong>-<strong>old</strong> Boy<strong>Who</strong> <strong>Has</strong> Congenital Heart DiseaseCase 3: Fever, Pharyngitis, and Chest <strong>Pain</strong>in a 9-<strong>year</strong>-<strong>old</strong> BoyThe reader is encouraged to writepossible diagnoses for each case beforeturning to the discussion.The editors and staff of Pediatrics inReview find themselves in thefortunate position of having too manysubmissions for the Index of Suspicioncolumn. Our publication slots for Indexof Suspicion are filled through 2013.Because we do not think it is fair todelay publication longer than that, wehave decided not to accept new casesfor the present. We will make anannouncement in Pediatrics in Reviewwhen we resume accepting new cases.We apologize for having to take thisstep, but we wish to be fair to allauthors. We are grateful for yourinterest in the journal.Author DisclosureDrs Stewart, Piteau, Storr, MacKenzie,Hartsell, and Nagappan have disclosedno financial relationships relevant tothis article. This commentary does notcontain a discussion of anunapproved/investigative use ofa commercial product/device.Case 1PresentationA previously healthy 23-month-<strong>old</strong>girl presents with fever and a limp.She has had fever every other dayfor the past 2 months. Over the past2 weeks, she has been favoring herright leg and occasionally complainsof leg pain with ambulation. Today’sclinic visit was prompted by an abruptrefusal to bear weight on the left leg.Review of systems is notable for subjectiveweight loss, chills, and nightsweats during the past month. Her immunizationsare up to date, and she hasno recent travel or exposure history.She takes no medications.On physical examination, the girlis alert but fussy and has growth parameterswithin normal limits. Sheis afebrile; her heart rate is 164 beatsper minute, respiratory rate is 28 breathsper minute, and blood pressure whilecrying is 158/91 mm Hg. Her leftlower extremity is held in extensionand external rotation; she will not allowabduction of her hip. There is norash, swelling, or warmth over the hipor knee joints bilaterally. She will notbear any weight on the left leg, butshe moves the right leg spontaneously.She has normal results on neurovascularexamination. A few scattered ecchymosesare noted on both legs. Theresults of the remainder of the physicalexamination are normal.Laboratory studies reveal a whiteblood cell count of 5.9 10 3 /mL,without any immature cells, hemoglobinlevel of 8 g/dL, and platelets of60 10 3 /mL. Her C-reactive proteinlevel is 5.7 mg/dL, and erythrocytesedimentation rate is 90 mm/h. Radiographsof the lower extremities showdemineralization and mottling at theleft femoral metaphysis. Additional laboratoryand imaging studies reveal thediagnosis.Case 2PresentationA 17-<strong>year</strong>-<strong>old</strong> boy with congenitalheart disease (CHD) presents witha fever and fatigue. His past medicalhistory includes a ventricular septaldefect (VSD) with aortic regurgitationand distichiasis (double set ofeyelashes arising from the meibomianglands) (Fig 1). Despite closure of hisVSD, he developed progressive aorticinsufficiency requiring multiple surgeries.His eyelashes were removedat 7 <strong>year</strong>s of age because of persistentconjunctival irritation due to the distichiasis.After his last cardiac surgical(Ross) procedure, he developed chronicprogressive right-sided pitting edemabelow the knee to the midfoot, withoutevidence of thrombosis.Physical examination reveals a temperatureof 37°C, heart rate of 90 beatsper minute, and blood pressure of99/61 mm Hg. The right eye showstwo aberrant eyelashes in the uppereyelid. His cardiovascular exam revealsa grade II/VI systolic ejection murmurheard loudest at the pulmonic area. Hisright leg is edematous, cool, and nontender.The rest of the physical findingsare normal.His complete blood cell count isnormal, and blood cultures are pending.<strong>An</strong> echocardiogram shows a successfulvalvular repair and no evidenceof endocarditis. Consultation with a specialistprovides the explanation for hisCHD, distichiasis, and lymphedema.Downloaded from http://pedsinreview.aappublications.org/ by Enrique Mendoza-Lopez on July 2, 2012Pediatrics in Review Vol.33 No.7 July 2012 327
index of suspicionFigure 1. This patient has a double set ofeyelashes (distichiasis). Photo courtesy ofPOSSUM-web 2009. Ó Murdoch ChildrensResearch Institute.Case 3PresentationA 9-<strong>year</strong>-<strong>old</strong> boy presents to theemergency department with 7 days offever to a height of 103°F (39.4°C)and night sweats. He complains alsoof sore throat, rhinorrhea, cough, decreasedappetite, fatigue, and chestpain, which is sharp and is locatedabove the sternum. Over the week,the rhinorrhea and cough have improved,but the fever, sore throat, andchest pain have worsened. He deniesdysphagia, but is spitting frequently.He also notes a change in his voice.On physical examination, the boyis not in acute distress. His temperatureis 103.1°F (39.6°C), respirationsare 18 breaths per minute, heart rateis 128 beats per minute, and bloodpressure is <strong>11</strong>3/72 mm Hg. He hasmild erythema of the pharynx withoutexudates. His neck is supple withoutlymphadenopathy. He has circulardesquamation surrounding his anus,w4 cm in diameter, with flaking skinon his scrotum; his palms are desquamatingin thick sheets. The remainingfindings on examination are normal.His white blood cell count is 30.7 10 3 /mL (87% neutrophils, 9% lymphocytes),hemoglobin level is <strong>11</strong>.1 g/dL,platelet count is 4<strong>11</strong> 10 3 /mL, sodiumlevel is 129 mEq/L, chloridelevel is 92 mEq/L, total serum proteinconcentration is 7.4 g/L, and albuminlevel is 2.2 g/L. A rapid streptococcalantigen test is positive. Chest radiographshows an w5.1 6.6 cm homogeneousopacity in the medialaspect of the right upper chest, extendingfrom the level of the superior hilumup toward the apex (Fig 2). <strong>An</strong> additionalimaging study reveals thediagnosis.Case 1DiscussionInitial radiographs of the left lowerextremity were concerning for an infectiousor an oncologic process. Thegirl’s serum lactate dehydrogenaselevel was 1,627 units/L, serum uricacid level was 6.3 mg/dL, and urinevanillylmandelic acid and homovanillicacid catecholamine levels were 466mg/g and 316 mg/g, respectively.This pattern was strongly suggestiveof an oncologic sympathomimeticprocess.Computed tomography (CT) scanof the chest/abdomen/pelvis was obtained,which showed a thoracic paraspinoussoft tissue mass with internalcalcifications as well as scattered lyticlesions of the inferior sternum, leftscapula, L1 and L3 vertebrae, T3and T4 vertebrae, iliac wings, andproximal femurs, in addition to a hepaticlesion, a pattern suggestive ofa metastatic small round blue cellFigure 2. Chest radiograph showing anw5.1 3 6.6 cm homogenous opacity inthe medial aspect of the right upperchest extending from the level of thesuperior hilum up toward the apex.tumor. Bone marrow and tumor biopsyshowed a poorly differentiatedneuroblastoma with unfavorable histologyconsistent with a stage 4 neuroblastoma.The results of the bloodcultures and tuberculin skin test werenegative.Differential DiagnosisFever and limp has a broad differentialdiagnosis, including infection, trauma,and malignancy, as well as inflammatory,orthopedic, and hematologic conditions.A thorough history is essentialfor discerning which laboratory and imagingstudies to pursue. Processes thatrequire immediate treatment shouldbe ruled out first and include septic arthritisand osteomyelitis. Acute onsetof limp suggests trauma or infection,whereas presentations with an insidiousonset are more suggestive of inflammatoryprocesses, such as rheumatologicand oncologic diseases.The ConditionEmbryonal cancers of the peripheralsympathetic nervous system encompassa spectrum of tumors with variabledegrees of neural differentiation.Neuroblastomas are undifferentiated,small, round, blue cell tumors; theyare the third most common pediatriccancer. Median age at diagnosis is2 <strong>year</strong>s of age, and 90% of cases arediagnosed by 5 <strong>year</strong>s of age. Neuroblastomais the most commonly diagnosedtumor in infants, comprisingw34% of neonatal malignancies. Theclinical and morphologic spectrum ofneuroblastoma is wide. Presentationvaries significantly and reflects the siteof sympathetic nervous system tissueaffected, as well as the degree of tumorinvasion.The most common preliminarysite of tumor invasion is the abdomen,either in the adrenals or retroperitonealsympathetic ganglia. In one-thirdof cases, neuroblastoma originatesfrom the cervical, thoracic, or pelvic328 Pediatrics in Review Vol.33 No.7 July 2012Downloaded from http://pedsinreview.aappublications.org/ by Enrique Mendoza-Lopez on July 2, 2012
index of suspicionganglia; in these cases, the disordermay present with Horner syndromeand neurologic symptoms of spinalcord compression. Paraneoplastic syndromesalso may develop and includesigns of opsomyoclonus, ataxia, andsecretory diarrhea. Rarely, infants maydevelop a unique type of neuroblastoma(stage 4s) that includes subcutaneoustumor nodules, extensive liver involvement,marrow disease, and a small primarytumor without bony involvement.Diagnosis is made through imagingstudies, which highlight mass andmetastases, as well as elevated urinehomovanillic acid and vanillylmandelicacid levels. Pathologic diagnosis isconfirmed by tissue biopsy or bydemonstrating neuroblasts in bonemarrow biopsy. Nuclear imaging modalities,such as positron emissiontomography and iodine 123 metaiodobenzylguanidine(I-123 MIBG),often are used to define better the extentof disease.Management and PrognosisThe overall 5-<strong>year</strong> survival for neuroblastomadepends on the stage of diseaseat diagnosis. Children with stage1 or 2 disease have long-term survivalrates >80%, whereas children withstage4diseasehaveonlya30%chanceof long-term survival. The most importantclinical and biological prognosticfactors currently used to determinetreatment are the age of the patient atdiagnosis, stage of disease, MYCgene status, and histologic factors.The mainstays of therapy dependon prognostic factors but incorporatesurgical resection, chemotherapy,and radiation. Newer therapies includemonoclonal antibody therapy and stemcell transplant.This patient received chemotherapyfollowed by two autologous bonemarrow transplants. She is currentlyreceiving chemotherapy, and her mostrecent MIBG scan showed no evidenceof recurrent disease.Lessons for the Clinician• Many disease entities can presentwith acute limb pain in childhood.• The patient’s history and physicalexamination should help determineappropriate laboratory andradiographic studies.• Fever, severe pain, and weight losssuggest more serious causes of limbpain that should prompt immediateevaluation.(Eileen Stewart, MD, Department ofPediatrics, University of New Mexico,Albuquerque, NM)Case 2DiscussionEntry of the features of congenitalheart disease, distichiasis, and lymphedemainto an online genetics databasesuggested a possible diagnosisof lymphedema-distichiasis syndrome.A clinical geneticist agreed with thediagnosis, and a mutation in theFOXC2 gene confirmed lymphedemadistichiasissyndrome.The ConditionLymphedema-distichiasis syndromeclassically presents with late-onsetlymphedema (typically late childhoodor puberty) and distichiasis.The lymphedema occurs exclusivelyin the lower extremities, often isasymmetric, and can be unilateral;the severity varies among patients,and males usually present earlier thanfemales. Approximately 94% of affectedindividuals have distichiasis,which can cause photophobia, recurrentconjunctivitis, and corneal irritation.CHD occurs in 7% of patientsand may include VSD, atrial septaldefect, patent ductus arteriosus, andTetralogy of Fallot. Other associatedfeatures of the syndrome include varicoseveins, ptosis, cleft palate, spinalextradural cysts, and other ocularanomalies.DiagnosisThis diagnosis is made clinically andconfirmed by analysis of FOXC2 gene,which reveals a mutation in about95% of cases. There is clinical variabilitywith no apparent genotype/phenotype correlation. Lymphedemadistichiasissyndrome is inherited inan autosomal dominant manner. Theremay be no known family history ofdistichiasis because relatives may notbe aware of asymptomatic cases. Alternatively,the condition may be dueto a new mutation, found in w25%of affected patients.Differential DiagnosisLymphedema should be consideredas a cause of any peripheral edemathat lacks pain or inflammation. Lymphedemacan be difficult to differentiatefrom chronic venous insufficiencybecause both can have pitting edema(although lymphedema typically isnot of the pitting type). Distinguishingfeatures of lymphedema includea unilateral presentation, a squaredoffappearance of the foot, and initialpitting edema progressing to nonpitting,brawny edema. In the laterstages of lymphedema, the skin becomeshyperkeratotic, hyperpigmented,and papillomatous or verrucous. TheKaposi-Stemmer sign, in which an examineris unable to pinch a f<strong>old</strong> of skinat the dorsal base of the second toe, isindicative of lymphedema.Lymphedema is classified as eitherprimary or secondary. In primarylymphedema, the dysfunction is causedby congenital hypoplasia or aplasia ofthe lymphatic vessels or by valvular incompetence,and the clinical changesmay not become apparent until laterin life. Primary lymphedema can be dividedinto three categories based onthe age of onset of the edema. Congenitallymphedema (Milroy disease)manifests from birth to 1 <strong>year</strong> of ageand is due to anaplastic lymphaticchannels. The lower-extremity edemaDownloaded from http://pedsinreview.aappublications.org/ by Enrique Mendoza-Lopez on July 2, 2012Pediatrics in Review Vol.33 No.7 July 2012 329
index of suspicionis bilateral, pitting, and nonpainful.Lymphedema praecox (Meige disease)manifestsfromage1to35<strong>year</strong>sandisthe most common form of primarylymphedema. This disorder is due tohypoplastic lymphatic channels. Theedema is unilateral, more common inthe distal extremity, and presents oftenat puberty. Lymphedema tardamanifests after age 35 <strong>year</strong>s and isthought to be due to a defect in thelymphatic valves. Primary lymphedemaalso is associated with genetic syndromes,including Turner and Noonansyndromes, as well as with rarer disorderssuch as lymphedema-distichiasissyndrome.In secondary lymphedema, thereis blockage of the lymphatic systemor disruption of the lymphatic drainagebecause of recurrent attacks oflymphangitis or infections with filariasis,malignancy, obesity, surgery, ortrauma.ManagementOnce the diagnosis of lymphedemadistichiasisis made, further evaluationis recommended to establish theextent of the disease. The followingsteps should be considered (the timingof which will depend on the initialpresentation): referral to an ophthalmologistfor slit lamp examination,physical examination to identify a cardiaclesion or the complication ofcellulitis, echocardiography, isotopelymphoscintigraphy to document theunderlying abnormality of the lymphaticsystem, and referral to a geneticistfor counseling.The goal in managing primarylymphedema is to restore functionand prevent deterioration. A multidisciplinaryteam is necessary for optimalmanagement. Initial treatmentincludes the use of compression stockings,multilayer bandages, or pneumaticpumps; leg elevation; and skincare and debridement to prevent cellulitis,if needed. In cases of recurrentcellulitis or lymphangitis, long-termantibiotic therapy with penicillin orcephalosporins should be considered.Some pharmacologic therapieshave proven to be effective in treatinglymphedema, including benzopyrenes,oral and topical retinoids, and topicalemollients and keratolytics. Diureticsare not effective in treating lymphedema.Surgery is reserved for casesresistant to medical therapy. Ophthalmologyreferral is indicated for themanagement of the distichiasis, whichcan be conservative management withlubrication or more definitive surgicalmanagement.This patient’s lymphedema currentlyis well controlled, his distichiasisis asymptomatic after surgical repair,and his last echocardiography showednormal heart size and function.PrognosisLower-extremity lymphedema canprogress if uncontrolled and can bea disabling disease with functional,cosmetic, and psychological consequences.The severity of the edemais variable in lymphedema-distichiasissyndrome. A multidisciplinary teamapproach, in combination with patienteducation and compliance, can improveoutcome. Prognosis dependsalso on associated comorbidities.Lessons for the Clinician• A high level of suspicion for a geneticsyndrome is required in thepresence of a rare anomaly.• The presence of two major anomaliessignificantly increases the likelihoodof an underlying geneticcondition. Especially with respectto the many rare diseases thatmanifest phenotypic variability,it is important to have a lowthresh<strong>old</strong> for considering geneticsyndrome.• Practitioners also should be familiarwith Web-based databasesand regional clinical geneticsresources.(Shalea Piteau, MD, Michael Storr, MD,Jennifer MacKenzie, MD, KingstonGeneral Hospital, Kingston, Canada)Case 3DiscussionContrast-enhanced CT scan of thechest reveals an extensive fluid collectionin the posterior mediastinumsuggestive of abscess (Fig 3).Differential DiagnosisThe differential diagnosis of the patient’sinitial symptoms includedpharyngitis, Ludwig angina, tonsillitis,and pneumonia. However, once themediastinal mass was seen on chest radiograph,the differential broadenedto include neoplasm, cystic lesions,vascular abnormalities, or infection.Further differentiation in such a casewould be based on the location ofthe mass within the mediastinum (anterior,middle, or posterior) and almostalways would require furtherevaluation with CT. This patient ultimatelywas found to have a posteriormediastinal abscess surrounding theesophagus and abutting the trachea.Clinical Course andManagementThe patient was admitted and startedon intravenous ampicillin/sulbactamFigure 3. Contrast-enhanced CT scan ofthe chest demonstrating an extensivefluid collection in the posterior mediastinumsuggestive of an abscess.330 Pediatrics in Review Vol.33 No.7 July 2012Downloaded from http://pedsinreview.aappublications.org/ by Enrique Mendoza-Lopez on July 2, 2012
index of suspicionand vancomycin. The following day,he developed a scarlatiniform rash.Later the same morning, he underwentsurgical drainage via dorsal thoracotomy.Surgeons discovered avery large abscess that extended fromhis posterior mediastinum and surroundedhis esophagus, trachea, andportions of the aortic arch. Therewas no intraoperative evidence of extensionto the neck. Cultures sentfrom the abscess grew group A Streptococcus.The desquamation of skinfrom his palms, anal region, andscrotum was due to streptococcalerythrogenic toxin that also causedthe scarlatiniform rash. Drains wereplaced in the abscess space and removedon hospital day 4. He was dischargedon hospital day 5 to complete a 14-daycourse of amoxicillin/clavulanate.The ConditionDescending mediastinitis is a termdefined by Estrera et al in 1983 asacute mediastinitis originating froman oropharyngeal infection. (1) Theauthors developed criteria for diagnosisof mediastinitis that include(1) clinical symptoms of severe oropharyngealinfection, (2) demonstrationof characteristic radiographicfeatures of mediastinitis, (3) documentationof mediastinitis at operation orpostmortem examination or both,and (4) establishment of a relationshipbetween oropharyngeal infection anddevelopment of the mediastinal process.Descending mediastinitis is a rare,but potentially life-threatening complicationof infections that spreadthrough the potential spaces formedby fascial planes extending from thehead and neck to the thorax.The initial clinical presentation includespain, fever, and swelling at theprimary site of infection. Early in thedevelopment of mediastinitis, initialcomplaints may include chest painand odynophagia (painful swallowing).Vocal change may be present.Respiratory distress and stridor arepossible when the infection causes airwayimpingement. As the disease progresses,physical examination findingsmay include the Hamman sign, acrunching raspy sound heard overthe precordium with the heart beat,or crepitus in the supraclavicular region.<strong>Left</strong> unrecognized, mediastinitismay progress to overwhelming sepsisand cardiovascular compromise.The most common predisposingcondition involves extension of a headand neck infection (pharyngitis, tonsillitis,sinusitis, parotitis, epiglottitis,Ludwig angina, and Lemierre syndrome).Oropharyngeal or cervicalabscesses, including retropharyngealabscesses, odontogenic abscesses, andperitonsillar abscesses are all leadingcauses. Trauma, surgery, or instrumentationmay directly translocatebacteria from the oropharynx intothe retropharyngeal space. The infectiontravels down the fascial planesof the neck via adjacent lymph nodesinto the superior mediastinum or intothe posterior mediastinum if the dangerspace, the space between the alarand prevertebral fascia, is involved.Mediastinitis rarely is due to spreadfrom distant sites, although cases secondaryto pneumonia, cellulitis, subphrenicabscess, or osteomyelitis ofthe rib, sternum, clavicle, or vertebraehave been described. The most commonorganisms implicated in thesesuppurative infections include groupA streptococci and common anaerobicoropharyngeal flora. Infections due tomethicillin-resistant Staphylococcusaureus may begin to play a largerrole.Treatment and PrognosisDelayed diagnosis and inadequatetreatment have led to a high mortalityin acute descending mediastinitis.Because there are few early clinicalsigns, patients may present in extremisafter a rapid progression oftheir symptoms. Mortality ratesrange from 15% to 40%. Treatmentis initiated with broad-spectrumintravenous antibiotics directed towardthe usual causative organisms,but surgery often is the definitivetreatment.Lessons for the Clinician• Descending mediastinitis is a rarebut potentially life-threateningcomplication of head and neckinfections that travel down fascialplanes to the mediastinum.• Descending mediastinitis shouldbe suspected when there are symptomsout of proportion to a simplepharyngeal or odontogenic infectionor in a patient who has recentlyundergone surgery or proceduresinvolving the oropharyngeal space.• Radiography of the chest may benegative early in the course. Earlycontrast CT scan of the chest is therecommended diagnostic imagingstudy in the appropriate context.• Initial therapy with broad-spectrumantibiotics is important, but earlysurgical intervention may benecessary.(<strong>An</strong>gela Hartsell, MD, MPH, SureshNagappan, MD, Moses Cone Hospital,Greensboro, NC)Reference1. Estrera AS, Landay MJ, Grisham JM,Sinn DP, Platt MR. Descending necrotizingmediastinitis. Surg Gynecol Obstet. 1983;157(6):545–552To view Suggested Reading lists, forthese cases, visit the July issue at http://pedsinreview.aappublications.org andclick on “Index of Suspicion.”Downloaded from http://pedsinreview.aappublications.org/ by Enrique Mendoza-Lopez on July 2, 2012Pediatrics in Review Vol.33 No.7 July 2012 331
in briefIn BriefColicGail M. Cohen, MD, MSLaurie W. Albertini, MDWake Forest University School ofMedicineWinston-Salem, NCAuthor DisclosureDrs Cohen, Albertini, and Serwint havedisclosed no financial relationshipsrelevant to this In Brief. Thiscommentary does not containa discussion of an unapproved/investigative use of a commercialproduct/device.Paroxysmal Fussing in Infancy,Sometimes Called “Colic.” Wessel MA,CobbJC,JacksonEB,HarrisGS,DetwilerAC. Pediatrics. 1954;14:421–435A Systematic Review of Treatmentsfor Infant Colic. Garrison MM,Christakis DA. Pediatrics. 2000;106:184–190Focus on Infantile Colic. Savino F. ActaPaediatrica. 2007;96:1259–1264Use of Soy Protein–Based Formulas inInfant Feeding. Bhatia J, Greer F.Pediatrics. 2008;121:1062–1068Nutritional Supplements and OtherComplementary Medicines forInfantile Colic: A Systematic Review.Perry R, Hunt K, Ernst E. Pediatrics.20<strong>11</strong>;127:720–733If we understood the mechanism of infantcolic, life would be so much easierfor parents and pediatricians. Being anew parent is difficult, but being theparent of a fussy infant is infinitelymore challenging. <strong>An</strong> estimated 10%to 26% of infants experience colic,which was defined by Wessel in hisclassic 1954 article as occurring in anotherwise healthy infant who cries for>3 hours per day, >3 days per week,for >3 weeks in duration. Colic beginsduring the second week of life, peaksat 6 weeks, and resolves between 12and 16 weeks. It is equally common inboth breast- and bottle-fed infants. Althoughcrying is normal for all infants,averaging 2.2 hours per day, those withcolic cry excessively, are more difficultto console, have disrupted sleep, andare the source of much parental anxiety.Mothers of colicky infants are athigher risk for postpartum depressionand are more likely to stop breastfeedingearly. Infants who are excessivelyfussy are at higher risk for child abuse.There is no racial, socioeconomic, orgender prevalence for colic.The diagnosis of colic is made byhistory and is a diagnosis of exclusion.Colicky infants present with episodicperiods of inconsolable crying, most oftenin the late afternoon and evening.These periods of fussiness are not associatedwith hunger or discomfort, andthe infants are otherwise normal. Laboratorytests and imaging are not necessaryto make the diagnosis. <strong>An</strong> organiccause can be found in
in briefapnea and seizures, and its use is contraindicatedin infants younger than6months.Complementary interventions are receivingmore attention for the treatmentof colic. These therapies includenatural and botanical products, probiotics,and manipulative therapies. Asystematic review of these modalitieswas published in 20<strong>11</strong> and found that,with the exception of a few limited studies,available data do not yet support theroutine use of any of these therapies.Although the etiology and thus effectivetreatment for colic remains elusive,it is a self-limited process withno long-term adverse effects. Parentsneed to be reassured that they havenormal, healthy infants. Presenting parentswith simple strategies to calm andsoothe their infants, and themselves,until this common but difficult time intheir child’s life passes is key.Comment: If you have ever been inthe presence of an infant with colic,you know how difficult it can be. Theheart-wrenching cries instill in all ofus—parents, family members, and pediatriciansalike—the impulse to wantto comfort and make the infant feelbetter. No wonder everyone seeks acure. But this search can lead to anever-ending cycle of trying medications,dietary manipulations, behavioralstrategies, and nutritional and complementarysupplements. Helping parentsto understand that colic is a self-limitedcondition and developing strategies toenhance their self-esteem in parentingare critical; however, the more severethesymptoms,themoreintensemustbe the support.Isn’t it amazing to know that colichas been described since biblical times,but we still do not truly understandits etiology or effective treatments?Systematic reviews on the topic reveal>1700 articles and abstracts aboutcolic that have been published, yetthere are few randomized trials, andthose that have been published spanmultiple treatment options of pharmaceutical,dietary, behavioral, and complementaryinterventions. Significantmethodologic flaws have hampered researchin this area, and future studiesneed to focus on a research agendato ensure the use of a consistent definitionof colic (Wessel criteria), objectiveoutcome measures, and sufficientsample size and power to determinedifferences. Time will tell, but perhapscolic will be one entity that will remainelusive, and our current strategies ofsupport and reassurance may be thebest.Janet R. Serwint, MDConsulting Editor, In BriefMarijuanaDaniel R. Neuspiel, MD, MPHUniversity of North Carolina School ofMedicine Charlotte, NCAuthor DisclosureDrs Neuspiel and Serwint havedisclosed no financial relationshipsrelevant to this In Brief. Thiscommentary does not containa discussion of an unapproved/investigative use of a commercialproduct/device.Monitoring the Future. National Resultson Adolescent Drug Use: Overviewof Key Findings, 2010. Johnston LD,O’Malley PM, Bachman JG,Schulenberg JE. <strong>An</strong>n Arbor, MI:Institute for Social Research, TheUniversity of Michigan; 20<strong>11</strong>.Available at: http://monitoringthefuture.org/pubs/monographs/mtf-overview2010.pdf.Accessed August 18, 20<strong>11</strong>A Meta-analytic Review of School-Based Prevention for Cannabis Use.Porath-Waller AJ, Beasley E, BeirnessDJ. Health, Education & Behavior.2010;37:709–723InfoFacts: Marijuana. Bethesda, MD:National Institute on Drug Abuse,revised November 2010. Available at:http://www.drugabuse.gov/PDF/InfoFacts/Marijuana.pdf. AccessedAugust 18, 20<strong>11</strong>Testing for Drugs of Abuse in Childrenand Adolescents: Addendum—Testing in Schools and at Home.Committee on Substance Abuse.Pediatrics. 2007;<strong>11</strong>9:627–630The Role of Schools in Combating IllicitSubstance Abuse. Council on SchoolHealth and Committee on SubstanceAbuse. Pediatrics. 2007;120:1379–1384Marijuana (cannabis), the illicit drug usedmost frequently in the United States,may be smoked in cigarettes (joints, nails,reefers), pipes (bongs, bowls), or cigars(blunts); mixed with food; or brewed as atea. <strong>Has</strong>hish (hash), a potent resin of cannabis,also may be used as a sticky blackliquid (hash oil). Other street terms arepot, herb, weed, grass, widow, and ganja.The 2010 Monitoring the Futurestudy, which assesses adolescent substanceuse patterns, revealed that admittedlifetime marijuana use amongeighth, 10th, and 12th graders in theUnited States was 17.3%, 33.4%, and43.8%, respectively; daily use was 1.2%,3.3%, and 6.1%, respectively. Trends inuse have varied in recent decades.The primary active chemical in marijuanais THC (delta-9-tetrahydrocannabinol).Increased cultivation of sinsemillamade from buds of female cannabisplants has raised mean THC content from0.7% in the 1970s to 8.5% in 2008, withwide variability in dose. Street marijuanaDownloaded from http://pedsinreview.aappublications.org/ by Enrique Mendoza-Lopez on July 2, 2012Pediatrics in Review Vol.33 No.7 July 2012 333
in briefapnea and seizures, and its use is contraindicatedin infants younger than6months.Complementary interventions are receivingmore attention for the treatmentof colic. These therapies includenatural and botanical products, probiotics,and manipulative therapies. Asystematic review of these modalitieswas published in 20<strong>11</strong> and found that,with the exception of a few limited studies,available data do not yet support theroutine use of any of these therapies.Although the etiology and thus effectivetreatment for colic remains elusive,it is a self-limited process withno long-term adverse effects. Parentsneed to be reassured that they havenormal, healthy infants. Presenting parentswith simple strategies to calm andsoothe their infants, and themselves,until this common but difficult time intheir child’s life passes is key.Comment: If you have ever been inthe presence of an infant with colic,you know how difficult it can be. Theheart-wrenching cries instill in all ofus—parents, family members, and pediatriciansalike—the impulse to wantto comfort and make the infant feelbetter. No wonder everyone seeks acure. But this search can lead to anever-ending cycle of trying medications,dietary manipulations, behavioralstrategies, and nutritional and complementarysupplements. Helping parentsto understand that colic is a self-limitedcondition and developing strategies toenhance their self-esteem in parentingare critical; however, the more severethesymptoms,themoreintensemustbe the support.Isn’t it amazing to know that colichas been described since biblical times,but we still do not truly understandits etiology or effective treatments?Systematic reviews on the topic reveal>1700 articles and abstracts aboutcolic that have been published, yetthere are few randomized trials, andthose that have been published spanmultiple treatment options of pharmaceutical,dietary, behavioral, and complementaryinterventions. Significantmethodologic flaws have hampered researchin this area, and future studiesneed to focus on a research agendato ensure the use of a consistent definitionof colic (Wessel criteria), objectiveoutcome measures, and sufficientsample size and power to determinedifferences. Time will tell, but perhapscolic will be one entity that will remainelusive, and our current strategies ofsupport and reassurance may be thebest.Janet R. Serwint, MDConsulting Editor, In BriefMarijuanaDaniel R. Neuspiel, MD, MPHUniversity of North Carolina School ofMedicine Charlotte, NCAuthor DisclosureDrs Neuspiel and Serwint havedisclosed no financial relationshipsrelevant to this In Brief. Thiscommentary does not containa discussion of an unapproved/investigative use of a commercialproduct/device.Monitoring the Future. National Resultson Adolescent Drug Use: Overviewof Key Findings, 2010. Johnston LD,O’Malley PM, Bachman JG,Schulenberg JE. <strong>An</strong>n Arbor, MI:Institute for Social Research, TheUniversity of Michigan; 20<strong>11</strong>.Available at: http://monitoringthefuture.org/pubs/monographs/mtf-overview2010.pdf.Accessed August 18, 20<strong>11</strong>A Meta-analytic Review of School-Based Prevention for Cannabis Use.Porath-Waller AJ, Beasley E, BeirnessDJ. Health, Education & Behavior.2010;37:709–723InfoFacts: Marijuana. Bethesda, MD:National Institute on Drug Abuse,revised November 2010. Available at:http://www.drugabuse.gov/PDF/InfoFacts/Marijuana.pdf. AccessedAugust 18, 20<strong>11</strong>Testing for Drugs of Abuse in Childrenand Adolescents: Addendum—Testing in Schools and at Home.Committee on Substance Abuse.Pediatrics. 2007;<strong>11</strong>9:627–630The Role of Schools in Combating IllicitSubstance Abuse. Council on SchoolHealth and Committee on SubstanceAbuse. Pediatrics. 2007;120:1379–1384Marijuana (cannabis), the illicit drug usedmost frequently in the United States,may be smoked in cigarettes (joints, nails,reefers), pipes (bongs, bowls), or cigars(blunts); mixed with food; or brewed as atea. <strong>Has</strong>hish (hash), a potent resin of cannabis,also may be used as a sticky blackliquid (hash oil). Other street terms arepot, herb, weed, grass, widow, and ganja.The 2010 Monitoring the Futurestudy, which assesses adolescent substanceuse patterns, revealed that admittedlifetime marijuana use amongeighth, 10th, and 12th graders in theUnited States was 17.3%, 33.4%, and43.8%, respectively; daily use was 1.2%,3.3%, and 6.1%, respectively. Trends inuse have varied in recent decades.The primary active chemical in marijuanais THC (delta-9-tetrahydrocannabinol).Increased cultivation of sinsemillamade from buds of female cannabisplants has raised mean THC content from0.7% in the 1970s to 8.5% in 2008, withwide variability in dose. Street marijuanaDownloaded from http://pedsinreview.aappublications.org/ by Enrique Mendoza-Lopez on July 2, 2012Pediatrics in Review Vol.33 No.7 July 2012 333
in briefalso may be contaminated with a variety ofother drugs, toxins, and infectious agents.After smoking marijuana, THC rapidlypasses from the lungs to the bloodstreamand brain, where it binds to cannabinoidreceptors. Euphoria and otherbrain effects occur within seconds ofsmoking, peak in 15 to 30 minutes,andtaperover2to3hours.Theonsetof action after oral ingestion is 30 to90 minutes, with peak effect in 2 to3 hours and a duration of 4 to 12 hours.THC is highly lipid-soluble and has aserum half-life of w19 hours.Physiologic effects include transienttachycardia for up to 3 hours, increasedsitting blood pressure with abnormalorthostatic responses, and peripheralvasodilation. Regular marijuana smokingmay cause respiratory effects similar tothose caused by tobacco, including dailycough, more frequent lung infections,exacerbation of asthma, decreased pulmonaryfunction, and increased risk ofrespiratory tract cancer. Marijuanasmoke contains 50% to 70% more carcinogenichydrocarbons than tobaccosmoke, and marijuana users typically inhalemore deeply and longer, increasingcarcinogen exposure. Cannabis may reduceimmune function, and heavy usecauses reversible decreases in spermcount and motility in animals. Irregularovulation also has been noted. Shorttermbehavioral effects include memoryand learning deficits, distorted sensoryand time perception, problem-solvingdifficulties, and impaired coordination.Heavy marijuana use may exacerbatedepression, anxiety, personality disorders,and sleep disturbance. There maybe negative effects on intellectual,employment, and social skills. Memoryimpairment may last up to 1 month afterlast use. Students using marijuana havelower high school grades and graduationrates than do nonusing peers.Marijuana increases the risk of injury,unwanted and unprotected sex,and other drug use. Recreational dosesimpair driving as much as blood alcoholconcentrations of 0.07% to 0.1%. Nodeleterious effects of marijuana use onthe fetus have been confirmed.Long-term marijuana use may leadto addiction, with use continuing despiteinterference with family life, school,work, and recreation. Tolerance mayoccur after several days of regularuse. Withdrawal symptoms after heavyusepeakbyday4andresolveby2weeks. These symptoms include irritability,insomnia, malaise, drug craving,diaphoresis, night sweats, gastrointestinaldisturbances, and agitation.Screening for drug use and addictivesymptoms is part of comprehensiveadolescent care. <strong>An</strong>ticipatory guidanceshould include information about marijuana’saddictive potential; injury risk;and possible impairment of learning, socialization,and sexual function. Parentsshould be encouraged to rehearse strategiesto help teenagers avoid drug-usingsettings. Skills-based interventions inschools have helped increase drug knowledge,decision-making, self-esteem, andpeer pressure resistance and have ledto reduced marijuana use. Interventionsin nonschool settings, motivational interviewing,and some family interventionsalso may help prevent marijuana use.Marijuana and its metabolites are detectablein urine by enzyme-multipliedimmunoassay technique starting 1 hourafter smoking. The urine assay usuallyremains positive up to 10 days after infrequentuse and up to 30 days in heavyusers. Some urine tests may detect passiveinhalation of second-hand marijuanasmoke. False-positive results mayoccur from ingestion of nonsteroidalanti-inflammatory drugs; false-negativeresults may follow urine dilution oradulteration. Various urine and salivatests as well as adulterants to modifythe tests are available on the Internet.Care must be taken to avoid contamination,dilution, or substitution when obtainingurine samples for testing.The American Academy of Pediatrics(AAP) supports voluntary confidentialdrug testing to ensure that adolescentsseek care. Although parents may aska physician to test their teenagers forevidence of drug use, the AAP states:“Testing adolescents requires their consentunless (1) a patient lacks decisionmakingcapacity; or (2) there are strongmedical indications or legal requirementsto do so.” Although recent courtdecisions allow schools to perform randomdrug tests on middle and highschool students participating in extracurricularactivities, the AAP has opposedschool-based testing unless partof a funded, comprehensive approachto addressing substance abuse.Comment: Some of the most challengingencounters I have experiencedhave involved parents asking for drugtesting for their adolescent children.Working toward a trusting relationshipbetween the adolescent and parent iscritical, and negotiating these situationshas been enhanced by the AAPstatement on this topic: that the drugtestingprocedure should be done withthe adolescent’s consent, and positiveresults should be used as a mechanismto get treatment for the patient, not punishment.Facilitating this dialogue andhelping the adolescent patient know thatboth family and pediatrician have his orher best interests in mind are essential.Much controversy has arisen over legalizingmarijuana use. Those who are opposedworry that access will be easier forchildren and adolescents and enhanceunwanted drug use. Those who are in favorthink that the therapeutic potential ofpain relief, increase in appetite, and nauseacontrol could be beneficial for selectpatients with AIDS, cancer, and multiplesclerosis. More research is needed to determinethe best way to approach thispublic health issue in controlling distributionto those who would benefit medicallywhile minimizing risk from recreational use.Janet Serwint, MDConsulting Editor, In Brief334 Pediatrics in Review Vol.33 No.7 July 2012Downloaded from http://pedsinreview.aappublications.org/ by Enrique Mendoza-Lopez on July 2, 2012