PHARMACY
eip26-sep15
eip26-sep15
- No tags were found...
You also want an ePaper? Increase the reach of your titles
YUMPU automatically turns print PDFs into web optimized ePapers that Google loves.
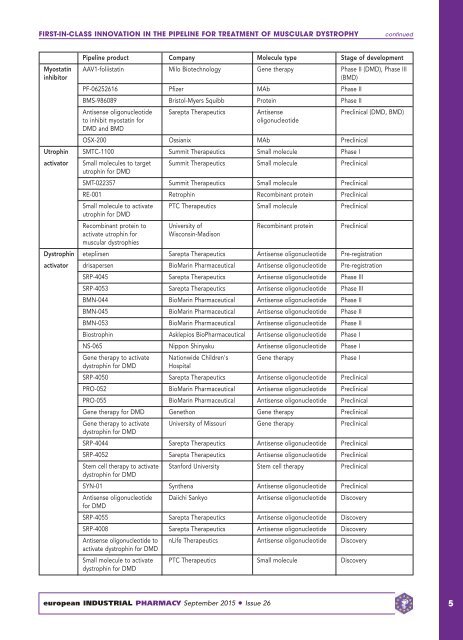
FIRST-IN-CLASS INNOVATION IN THE PIPELINE FOR TREATMENT OF MUSCULAR DYSTROPHY<br />
continued<br />
Pipeline product Company Molecule type Stage of development<br />
Myostatin AAV1-foliistatin Milo Biotechnology Gene therapy Phase II (DMD), Phase III<br />
inhibitor<br />
(BMD)<br />
PF-06252616 Pfizer MAb Phase II<br />
BMS-986089 Bristol-Myers Squibb Protein Phase II<br />
Antisense oligonucleotide Sarepta Therapeutics Antisense Preclinical (DMD, BMD)<br />
to inhibit myostatin for<br />
oligonucleotide<br />
DMD and BMD<br />
OSX-200 Ossianix MAb Preclinical<br />
Utrophin SMTC-1100 Summit Therapeutics Small molecule Phase I<br />
activator Small molecules to target Summit Therapeutics Small molecule Preclinical<br />
utrophin for DMD<br />
SMT-022357 Summit Therapeutics Small molecule Preclinical<br />
RE-001 Retrophin Recombinant protein Preclinical<br />
Small molecule to activate PTC Therapeutics Small molecule Preclinical<br />
utrophin for DMD<br />
Recombinant protein to University of Recombinant protein Preclinical<br />
activate utrophin for<br />
Wisconsin-Madison<br />
muscular dystrophies<br />
Dystrophin eteplirsen Sarepta Therapeutics Antisense oligonucleotide Pre-registration<br />
activator drisapersen BioMarin Pharmaceutical Antisense oligonucleotide Pre-registration<br />
SRP-4045 Sarepta Therapeutics Antisense oligonucleotide Phase III<br />
SRP-4053 Sarepta Therapeutics Antisense oligonucleotide Phase III<br />
BMN-044 BioMarin Pharmaceutical Antisense oligonucleotide Phase II<br />
BMN-045 BioMarin Pharmaceutical Antisense oligonucleotide Phase II<br />
BMN-053 BioMarin Pharmaceutical Antisense oligonucleotide Phase II<br />
Biostrophin Asklepios BioPharmaceutical Antisense oligonucleotide Phase I<br />
NS-065 Nippon Shinyaku Antisense oligonucleotide Phase I<br />
Gene therapy to activate Nationwide Children's Gene therapy Phase I<br />
dystrophin for DMD<br />
Hospital<br />
SRP-4050 Sarepta Therapeutics Antisense oligonucleotide Preclinical<br />
PRO-052 BioMarin Pharmaceutical Antisense oligonucleotide Preclinical<br />
PRO-055 BioMarin Pharmaceutical Antisense oligonucleotide Preclinical<br />
Gene therapy for DMD Genethon Gene therapy Preclinical<br />
Gene therapy to activate University of Missouri Gene therapy Preclinical<br />
dystrophin for DMD<br />
SRP-4044 Sarepta Therapeutics Antisense oligonucleotide Preclinical<br />
SRP-4052 Sarepta Therapeutics Antisense oligonucleotide Preclinical<br />
Stem cell therapy to activate Stanford University Stem cell therapy Preclinical<br />
dystrophin for DMD<br />
SYN-01 Synthena Antisense oligonucleotide Preclinical<br />
Antisense oligonucleotide Daiichi Sankyo Antisense oligonucleotide Discovery<br />
for DMD<br />
SRP-4055 Sarepta Therapeutics Antisense oligonucleotide Discovery<br />
SRP-4008 Sarepta Therapeutics Antisense oligonucleotide Discovery<br />
Antisense oligonucleotide to nLife Therapeutics Antisense oligonucleotide Discovery<br />
activate dystrophin for DMD<br />
Small molecule to activate PTC Therapeutics Small molecule Discovery<br />
dystrophin for DMD<br />
european INDUSTRIAL <strong>PHARMACY</strong> September 2015 • Issue 26<br />
5