Magazine – PDF - Cal Lab Magazine
Magazine – PDF - Cal Lab Magazine
Magazine – PDF - Cal Lab Magazine
You also want an ePaper? Increase the reach of your titles
YUMPU automatically turns print PDFs into web optimized ePapers that Google loves.
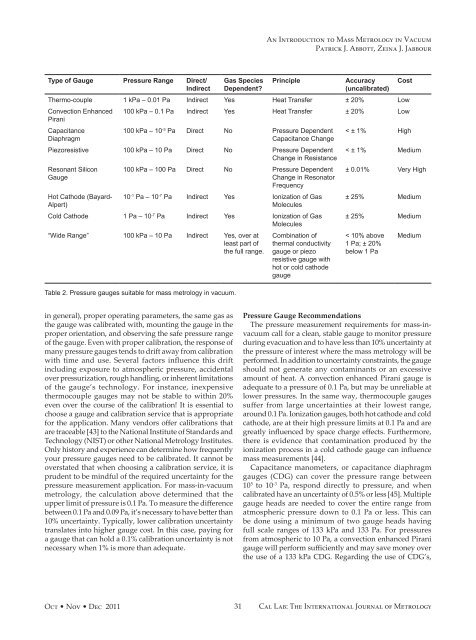
Type of Gauge Pressure Range Direct/<br />
Indirect<br />
in general), proper operating parameters, the same gas as<br />
the gauge was calibrated with, mounting the gauge in the<br />
proper orientation, and observing the safe pressure range<br />
of the gauge. Even with proper calibration, the response of<br />
many pressure gauges tends to drift away from calibration<br />
with time and use. Several factors influence this drift<br />
including exposure to atmospheric pressure, accidental<br />
over pressurization, rough handling, or inherent limitations<br />
of the gauge’s technology. For instance, inexpensive<br />
thermocouple gauges may not be stable to within 20%<br />
even over the course of the calibration! It is essential to<br />
choose a gauge and calibration service that is appropriate<br />
for the application. Many vendors offer calibrations that<br />
are traceable [43] to the National Institute of Standards and<br />
Technology (NIST) or other National Metrology Institutes.<br />
Only history and experience can determine how frequently<br />
your pressure gauges need to be calibrated. It cannot be<br />
overstated that when choosing a calibration service, it is<br />
prudent to be mindful of the required uncertainty for the<br />
pressure measurement application. For mass-in-vacuum<br />
metrology, the calculation above determined that the<br />
upper limit of pressure is 0.1 Pa. To measure the difference<br />
between 0.1 Pa and 0.09 Pa, it’s necessary to have better than<br />
10% uncertainty. Typically, lower calibration uncertainty<br />
translates into higher gauge cost. In this case, paying for<br />
a gauge that can hold a 0.1% calibration uncertainty is not<br />
necessary when 1% is more than adequate.<br />
Gas Species<br />
Dependent?<br />
Principle Accuracy<br />
(uncalibrated)<br />
Thermo-couple 1 kPa <strong>–</strong> 0.01 Pa Indirect Yes Heat Transfer ± 20% Low<br />
Convection Enhanced<br />
Pirani<br />
Capacitance<br />
Diaphragm<br />
100 kPa <strong>–</strong> 0.1 Pa Indirect Yes Heat Transfer ± 20% Low<br />
100 kPa <strong>–</strong> 10 -3 Pa Direct No Pressure Dependent<br />
Capacitance Change<br />
Piezoresistive 100 kPa <strong>–</strong> 10 Pa Direct No Pressure Dependent<br />
Change in Resistance<br />
Resonant Silicon<br />
Gauge<br />
Hot Cathode (Bayard-<br />
Alpert)<br />
100 kPa <strong>–</strong> 100 Pa Direct No Pressure Dependent<br />
Change in Resonator<br />
Frequency<br />
10 -1 Pa <strong>–</strong> 10 -7 Pa Indirect Yes Ionization of Gas<br />
Molecules<br />
Cold Cathode 1 Pa <strong>–</strong> 10 -7 Pa Indirect Yes Ionization of Gas<br />
Molecules<br />
“Wide Range” 100 kPa <strong>–</strong> 10 Pa Indirect Yes, over at<br />
least part of<br />
the full range.<br />
Table 2. Pressure gauges suitable for mass metrology in vacuum.<br />
An Introduction to Mass Metrology in Vacuum<br />
Patrick J. Abbott, Zeina J. Jabbour<br />
Combination of<br />
thermal conductivity<br />
gauge or piezo<br />
resistive gauge with<br />
hot or cold cathode<br />
gauge<br />
Pressure Gauge Recommendations<br />
The pressure measurement requirements for mass-invacuum<br />
call for a clean, stable gauge to monitor pressure<br />
during evacuation and to have less than 10% uncertainty at<br />
the pressure of interest where the mass metrology will be<br />
performed. In addition to uncertainty constraints, the gauge<br />
should not generate any contaminants or an excessive<br />
amount of heat. A convection enhanced Pirani gauge is<br />
adequate to a pressure of 0.1 Pa, but may be unreliable at<br />
lower pressures. In the same way, thermocouple gauges<br />
suffer from large uncertainties at their lowest range,<br />
around 0.1 Pa. Ionization gauges, both hot cathode and cold<br />
cathode, are at their high pressure limits at 0.1 Pa and are<br />
greatly influenced by space charge effects. Furthermore,<br />
there is evidence that contamination produced by the<br />
ionization process in a cold cathode gauge can influence<br />
mass measurements [44].<br />
Capacitance manometers, or capacitance diaphragm<br />
gauges (CDG) can cover the pressure range between<br />
10 5 to 10 -3 Pa, respond directly to pressure, and when<br />
calibrated have an uncertainty of 0.5% or less [45]. Multiple<br />
gauge heads are needed to cover the entire range from<br />
atmospheric pressure down to 0.1 Pa or less. This can<br />
be done using a minimum of two gauge heads having<br />
full scale ranges of 133 kPa and 133 Pa. For pressures<br />
from atmospheric to 10 Pa, a convection enhanced Pirani<br />
gauge will perform sufficiently and may save money over<br />
the use of a 133 kPa CDG. Regarding the use of CDG’s,<br />
Oct • Nov • Dec 2011 31 <strong>Cal</strong> <strong>Lab</strong>: The International Journal of Metrology<br />
Cost<br />
< ± 1% High<br />
< ± 1% Medium<br />
± 0.01% Very High<br />
± 25% Medium<br />
± 25% Medium<br />
< 10% above<br />
1 Pa; ± 20%<br />
below 1 Pa<br />
Medium