Create successful ePaper yourself
Turn your PDF publications into a flip-book with our unique Google optimized e-Paper software.
Step Two: solid ice melts<br />
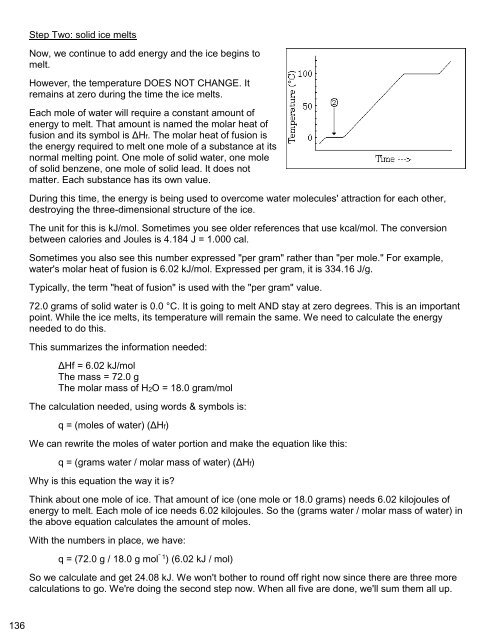
Now, we continue to add energy and the ice begins to<br />
melt.<br />
However, the temperature DOES NOT CHANGE. It<br />
remains at zero during the time the ice melts.<br />
Each mole of water will require a constant amount of<br />
energy to melt. That amount is named the molar heat of<br />
fusion and its symbol is ΔHf. The molar heat of fusion is<br />
the energy required to melt one mole of a substance at its<br />
normal melting point. One mole of solid water, one mole<br />
of solid benzene, one mole of solid lead. It does not<br />
matter. Each substance has its own value.<br />
During this time, the energy is being used to overcome water molecules' attraction for each other,<br />
destroying the three-dimensional structure of the ice.<br />
The unit for this is kJ/mol. Sometimes you see older references that use kcal/mol. The conversion<br />
between calories and Joules is 4.184 J = 1.000 cal.<br />
Sometimes you also see this number expressed "per gram" rather than "per mole." For example,<br />
water's molar heat of fusion is 6.02 kJ/mol. Expressed per gram, it is 334.16 J/g.<br />
Typically, the term "heat of fusion" is used with the "per gram" value.<br />
72.0 grams of solid water is 0.0 °C. It is going to melt AND stay at zero degrees. This is an important<br />
point. While the ice melts, its temperature will remain the same. We need to calculate the energy<br />
needed to do this.<br />
This summarizes the information needed:<br />
ΔHf = 6.02 kJ/mol<br />
The mass = 72.0 g<br />
The molar mass of H2O = 18.0 gram/mol<br />
The calculation needed, using words & symbols is:<br />
q = (moles of water) (ΔHf)<br />
We can rewrite the moles of water portion and make the equation like this:<br />
q = (grams water / molar mass of water) (ΔHf)<br />
Why is this equation the way it is?<br />
Think about one mole of ice. That amount of ice (one mole or 18.0 grams) needs 6.02 kilojoules of<br />
energy to melt. Each mole of ice needs 6.02 kilojoules. So the (grams water / molar mass of water) in<br />
the above equation calculates the amount of moles.<br />
With the numbers in place, we have:<br />
q = (72.0 g / 18.0 g mol¯1 ) (6.02 kJ / mol)<br />
So we calculate and get 24.08 kJ. We won't bother to round off right now since there are three more<br />
calculations to go. We're doing the second step now. When all five are done, we'll sum them all up.<br />
136