You also want an ePaper? Increase the reach of your titles
YUMPU automatically turns print PDFs into web optimized ePapers that Google loves.
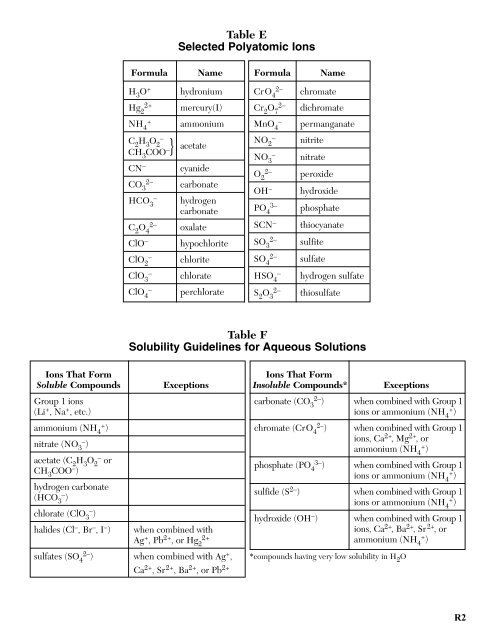
Table E<br />
Selected Polyatomic Ions<br />
Formula Name Formula Name<br />
H 3<br />
O +<br />
hydronium<br />
CrO 4<br />
2–<br />
chromate<br />
Hg 2<br />
2+<br />
mercury(I)<br />
Cr 2<br />
O 7<br />
2–<br />
dichromate<br />
NH 4<br />
+<br />
C 2<br />
H 3<br />
O<br />
–<br />
2 –}<br />
CH 3<br />
COO<br />
CN –<br />
CO 3<br />
2–<br />
HCO<br />
–<br />
3<br />
C 2<br />
O<br />
2–<br />
4<br />
ClO –<br />
ammonium<br />
acetate<br />
cyanide<br />
carbonate<br />
hydrogen<br />
carbonate<br />
oxalate<br />
hypochlorite<br />
MnO 4<br />
–<br />
NO<br />
–<br />
2<br />
NO<br />
–<br />
3<br />
O<br />
2–<br />
2<br />
OH –<br />
PO 4<br />
3–<br />
SCN –<br />
SO 3<br />
2–<br />
permanganate<br />
nitrite<br />
nitrate<br />
peroxide<br />
hydroxide<br />
phosphate<br />
thiocyanate<br />
sulfite<br />
ClO 2<br />
–<br />
chlorite<br />
SO 4<br />
2–<br />
sulfate<br />
ClO 3<br />
–<br />
chlorate<br />
HSO 4<br />
–<br />
hydrogen sulfate<br />
ClO 4<br />
–<br />
perchlorate<br />
S 2<br />
O 3<br />
2–<br />
thiosulfate<br />
Table F<br />
Solubility Guidelines for Aqueous Solutions<br />
Ions That Form<br />
Soluble Compounds<br />
Group 1 ions<br />
(Li + , Na + , etc.)<br />
ammonium (NH 4 + )<br />
nitrate (NO 3 – )<br />
acetate (C 2<br />
H 3<br />
O 2 – or<br />
CH 3<br />
COO – )<br />
hydrogen carbonate<br />
(HCO 3 – )<br />
chlorate (ClO 3 – )<br />
halides (Cl – , Br – , I – )<br />
Exceptions<br />
when combined with<br />
Ag + , Pb 2+ , or Hg 2<br />
2+<br />
sulfates (SO 4 2– ) when combined with Ag + ,<br />
Ca 2+ , Sr 2+ , Ba 2+ , or Pb 2+<br />
Ions That Form<br />
Insoluble Compounds*<br />
Exceptions<br />
carbonate (CO 3 2– ) when combined with Group 1<br />
ions or ammonium (NH 4 + )<br />
chromate (CrO 4 2– ) when combined with Group 1<br />
ions, Ca 2+ , Mg 2+ , or<br />
ammonium (NH 4 + )<br />
phosphate (PO 4 3– ) when combined with Group 1<br />
ions or ammonium (NH 4 + )<br />
sulfide (S 2– ) when combined with Group 1<br />
ions or ammonium (NH 4 + )<br />
hydroxide (OH – ) when combined with Group 1<br />
ions, Ca 2+ , Ba 2+ , Sr 2+ , or<br />
ammonium (NH 4 + )<br />
*compounds having very low solubility in H 2 O<br />
R2