2007_6_Nr6_EEMJ
You also want an ePaper? Increase the reach of your titles
YUMPU automatically turns print PDFs into web optimized ePapers that Google loves.
Draghici et al. /Environmental Engineering and Management Journal 6 (<strong>2007</strong>), 6, 497-503<br />
major source of Cu pollution was smelters that<br />
contributed vast quantities of Cu–S particulates to the<br />
atmosphere.<br />
a)<br />
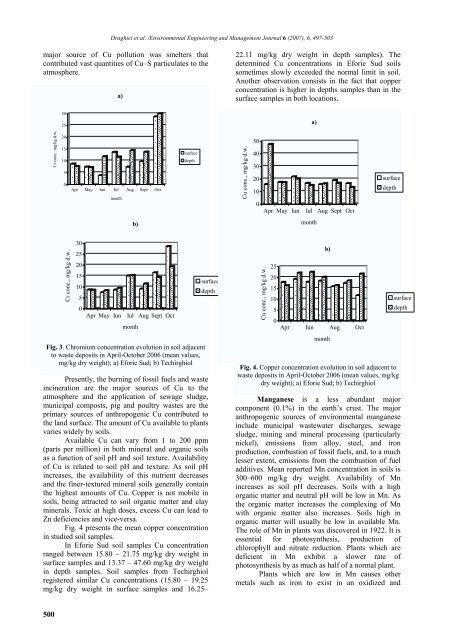
22.11 mg/kg dry weight in depth samples). The<br />
determined Cu concentrations in Eforie Sud soils<br />
sometimes slowly exceeded the normal limit in soil.<br />
Another observation consists in the fact that copper<br />
concentration is higher in depths samples than in the<br />
surface samples in both locations.<br />
30<br />
25<br />
a)<br />
Cr conc., mg/kg d.w.<br />
20<br />
15<br />
10<br />
5<br />
0<br />
Apr May Iun Iul Aug Sept Oct<br />
month<br />
surface<br />
depth<br />
Cu conc., mg/kg d.w.<br />
50<br />
40<br />
30<br />
20<br />
10<br />
0<br />
Apr May Iun Iul Aug Sept Oct<br />
surface<br />
depth<br />
b)<br />
month<br />
Cr conc., mg/kg d.w.<br />
30<br />
25<br />
20<br />
15<br />
10<br />
5<br />
0<br />
Apr May Iun Iul Aug Sept Oct<br />
month<br />
Fig. 3. Chromium concentration evolution in soil adjacent<br />
to waste deposits in April-October 2006 (mean values,<br />
mg/kg dry weight); a) Eforie Sud; b) Techirghiol<br />
surface<br />
depth<br />
Presently, the burning of fossil fuels and waste<br />
incineration are the major sources of Cu to the<br />
atmosphere and the application of sewage sludge,<br />
municipal composts, pig and poultry wastes are the<br />
primary sources of anthropogenic Cu contributed to<br />
the land surface. The amount of Cu available to plants<br />
varies widely by soils.<br />
Available Cu can vary from 1 to 200 ppm<br />
(parts per million) in both mineral and organic soils<br />
as a function of soil pH and soil texture. Availability<br />
of Cu is related to soil pH and texture. As soil pH<br />
increases, the availability of this nutrient decreases<br />
and the finer-textured mineral soils generally contain<br />
the highest amounts of Cu. Copper is not mobile in<br />
soils, being attracted to soil organic matter and clay<br />
minerals. Toxic at high doses, excess Cu can lead to<br />
Zn deficiencies and vice-versa.<br />
Fig. 4 presents the mean copper concentration<br />
in studied soil samples.<br />
In Eforie Sud soil samples Cu concentration<br />
ranged between 15.80 – 21.75 mg/kg dry weight in<br />
surface samples and 13.37 – 47.60 mg/kg dry weight<br />
in depth samples. Soil samples from Techirghiol<br />
registered similar Cu concentrations (15.80 – 19.25<br />
mg/kg dry weight in surface samples and 16.25–<br />
Cu conc., mg/kg d.w.<br />
25<br />
20<br />
15<br />
10<br />
5<br />
0<br />
Apr Iun Aug Oct<br />
month<br />
Fig. 4. Copper concentration evolution in soil adjacent to<br />
waste deposits in April-October 2006 (mean values, mg/kg<br />
dry weight); a) Eforie Sud; b) Techirghiol<br />
Manganese is a less abundant major<br />
component (0.1%) in the earth’s crust. The major<br />
anthropogenic sources of environmental manganese<br />
include municipal wastewater discharges, sewage<br />
sludge, mining and mineral processing (particularly<br />
nickel), emissions from alloy, steel, and iron<br />
production, combustion of fossil fuels, and, to a much<br />
lesser extent, emissions from the combustion of fuel<br />
additives. Mean reported Mn concentration in soils is<br />
300–600 mg/kg dry weight. Availability of Mn<br />
increases as soil pH decreases. Soils with a high<br />
organic matter and neutral pH will be low in Mn. As<br />
the organic matter increases the complexing of Mn<br />
with organic matter also increases. Soils high in<br />
organic matter will usually be low in available Mn.<br />
The role of Mn in plants was discovered in 1922. It is<br />
essential for photosynthesis, production of<br />
chlorophyll and nitrate reduction. Plants which are<br />
deficient in Mn exhibit a slower rate of<br />
photosynthesis by as much as half of a normal plant.<br />
Plants which are low in Mn causes other<br />
metals such as iron to exist in an oxidized and<br />
b)<br />
surface<br />
depth<br />
500