2007_6_Nr6_EEMJ
You also want an ePaper? Increase the reach of your titles
YUMPU automatically turns print PDFs into web optimized ePapers that Google loves.
- doped zeolite (Rivera-Garza et al., 2000).<br />
No major differences in the diffraction patterns<br />
were observed due to de presence of the low amount<br />
of silver into zeolite. However, traces of AgAlO 2<br />
(21.5°; 30.5°) were identified.<br />
Figs 3a and 3b illustrates the SEM images of<br />
natural and thermally treated Ag-doped zeolite.<br />
Obtaining and characterization of Romanian zeolite supporting silver ions<br />
a)<br />
Fig. 3a. SEM image of the natural zeolite<br />
b)<br />
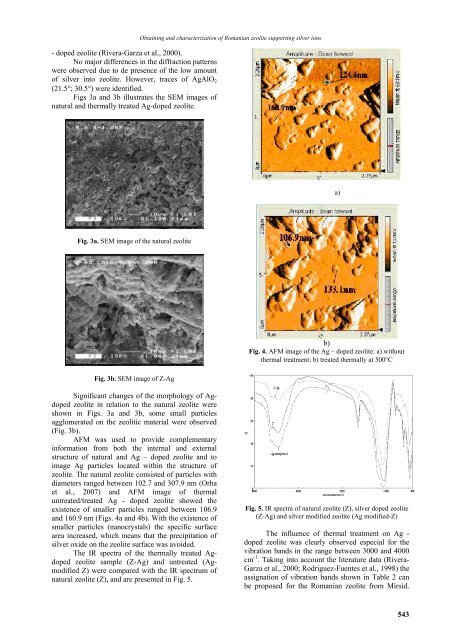
Fig. 4. AFM image of the Ag – doped zeolite: a) without<br />
thermal treatment; b) treated thermally at 500°C<br />
Fig. 3b. SEM image of Z-Ag<br />
Significant changes of the morphology of Agdoped<br />
zeolite in relation to the natural zeolite were<br />
shown in Figs. 3a and 3b, some small particles<br />
agglomerated on the zeolitic material were observed<br />
(Fig. 3b).<br />
AFM was used to provide complementary<br />
information from both the internal and external<br />
structure of natural and Ag – doped zeolite and to<br />
image Ag particles located within the structure of<br />
zeolite. The natural zeolite consisted of particles with<br />
diameters ranged between 102.7 and 307.9 nm (Orha<br />
et al., <strong>2007</strong>) and AFM image of thermal<br />
untreated/treated Ag - doped zeolite showed the<br />
existence of smaller particles ranged between 106.9<br />
and 160.9 nm (Figs. 4a and 4b). With the existence of<br />
smaller particles (nanocrystals) the specific surface<br />
area increased, which means that the precipitation of<br />
silver oxide on the zeolite surface was avoided.<br />
The IR spectra of the thermally treated Agdoped<br />
zeolite sample (Z-Ag) and untreated (Agmodified<br />
Z) were compared with the IR spectrum of<br />
natural zeolite (Z), and are presented in Fig. 5.<br />
Fig. 5. IR spectra of natural zeolite (Z), silver doped zeolite<br />
(Z-Ag) and silver modified zeolite (Ag modified-Z)<br />
The influence of thermal treatment on Ag -<br />
doped zeolite was clearly observed especial for the<br />
vibration bands in the range between 3000 and 4000<br />
cm -1 . Taking into account the literature data (Rivera-<br />
Garza et al., 2000; Rodriguez-Fuentes et al., 1998) the<br />
assignation of vibration bands shown in Table 2 can<br />
be proposed for the Romanian zeolite from Mirsid.<br />
543