2007_6_Nr6_EEMJ
You also want an ePaper? Increase the reach of your titles
YUMPU automatically turns print PDFs into web optimized ePapers that Google loves.
Chirila et al /Environmental Engineering and Management Journal 6 (<strong>2007</strong>), 6, 549-553<br />
Conversia NO x<br />
[%]<br />
100<br />
90<br />
80<br />
70<br />
60<br />
50<br />
40<br />
30<br />
20<br />
10<br />
0<br />
600 °C<br />
800 °C<br />
1000 °C<br />
150 200 250 300 350 400 450 500 550 600 650<br />
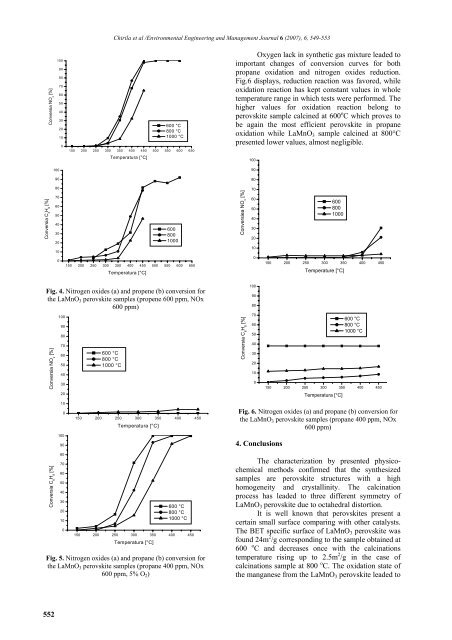
Oxygen lack in synthetic gas mixture leaded to<br />
important changes of conversion curves for both<br />
propane oxidation and nitrogen oxides reduction.<br />
Fig.6 displays, reduction reaction was favored, while<br />
oxidation reaction has kept constant values in whole<br />
temperature range in which tests were performed. The<br />
higher values for oxidation reaction belong to<br />
perovskite sample calcined at 600 o C which proves to<br />
be again the most efficient perovskite in propane<br />
oxidation while LaMnO 3 sample calcined at 800°C<br />
presented lower values, almost negligible.<br />
Temperatura [°C]<br />
100<br />
100<br />
90<br />
90<br />
80<br />
Conversia C 3<br />
H 6<br />
[%]<br />
80<br />
70<br />
60<br />
50<br />
40<br />
30<br />
20<br />
10<br />
0<br />
600<br />
800<br />
1000<br />
150 200 250 300 350 400 450 500 550 600 650<br />
Temperatura [°C]<br />
Conversiea NO x<br />
[%]<br />
70<br />
60<br />
50<br />
40<br />
30<br />
20<br />
10<br />
0<br />
600<br />
800<br />
1000<br />
150 200 250 300 350 400 450<br />
Temperature [°C]<br />
Fig. 4. Nitrogen oxides (a) and propene (b) conversion for<br />
the LaMnO 3 perovskite samples (propene 600 ppm, NOx<br />
600 ppm)<br />
Conversia NO x<br />
[%]<br />
Conversia C 6<br />
H 8<br />
[%]<br />
100<br />
90<br />
80<br />
70<br />
60<br />
50<br />
40<br />
30<br />
20<br />
10<br />
0<br />
100<br />
90<br />
80<br />
70<br />
60<br />
50<br />
40<br />
30<br />
20<br />
10<br />
0<br />
600 °C<br />
800 °C<br />
1000 °C<br />
150 200 250 300 350 400 450<br />
Temperatura [°C]<br />
600 °C<br />
800 °C<br />
1000 °C<br />
150 200 250 300 350 400 450<br />
Temperatura [°C]<br />
Fig. 5. Nitrogen oxides (a) and propane (b) conversion for<br />
the LaMnO 3 perovskite samples (propane 400 ppm, NOx<br />
600 ppm, 5% O 2 )<br />
Conversia C 6<br />
H 8<br />
[%]<br />
100<br />
90<br />
80<br />
70<br />
60<br />
50<br />
40<br />
30<br />
20<br />
10<br />
0<br />
600 °C<br />
800 °C<br />
1000 °C<br />
150 200 250 300 350 400 450<br />
Temperatura [°C]<br />
Fig. 6. Nitrogen oxides (a) and propane (b) conversion for<br />
the LaMnO 3 perovskite samples (propane 400 ppm, NOx<br />
600 ppm)<br />
4. Conclusions<br />
The characterization by presented physicochemical<br />
methods confirmed that the synthesized<br />
samples are perovskite structures with a high<br />
homogeneity and crystallinity. The calcination<br />
process has leaded to three different symmetry of<br />
LaMnO 3 perovskite due to octahedral distortion.<br />
It is well known that perovskites present a<br />
certain small surface comparing with other catalysts.<br />
The BET specific surface of LaMnO 3 perovskite was<br />
found 24m 2 /g corresponding to the sample obtained at<br />
600 o C and decreases once with the calcinations<br />
temperature rising up to 2.5m 2 /g in the case of<br />
calcinations sample at 800 o C. The oxidation state of<br />
the manganese from the LaMnO 3 perovskite leaded to<br />
552