Dry Eye 2016
Create successful ePaper yourself
Turn your PDF publications into a flip-book with our unique Google optimized e-Paper software.
SPECIAL FEATURE: DRY EYE<br />
Contact lenses and dry eye<br />
<strong>Dry</strong> eye is one of the most common<br />
conditions seen by eye care practitioners<br />
around the world. It is estimated that<br />
25% of patients will report symptoms of dry<br />
eye¹. For our contact lens (CL) patients this<br />
frequency is likely higher due to the effects that<br />
an in situ contact lens can have on the tear film<br />
and ocular tissue. Reported rates of contact lens<br />
discontinuation internationally vary from 15%<br />
to 31%². Consistently, discomfort and dryness<br />
are rated as the top reasons for this dropout 3 4 5,6 .<br />
However, certain types of contact lenses can also<br />
be an important management option for patients<br />
with mild to severe dry eye. This article will briefly<br />
explore why contact lenses cause dry eye, explain<br />
what can be done to reduce the incidence of dry<br />
eye symptoms in contact lens wearers and describe<br />
some of the contact lens options available to<br />
patients with existing dry eye disease.<br />
Why do CLs cause dryness?<br />
In 2013 the Tear Film and Ocular Surface Society<br />
(TFOS) international workshop on contact lens<br />
discomfort carried out a robust investigation into<br />
this question. Helmed by our very own Associate<br />
Professor Jennifer Craig, the TFOS report on tear<br />
film stability in contact lens wearers suggested<br />
that contact lens induced dryness stems from the<br />
alteration of the tear film into pre- and post-lens<br />
layers (Fig 1.) 7 . The resulting pre-lens tear film has<br />
reduced lipid layer thickness, reduced tear volume<br />
and an increased evaporation rate compared<br />
to the normal tear film 8 . As a result of this<br />
compromised tear film, previously asymptomatic<br />
patients may begin to experience discomfort and<br />
dry eye symptoms 9 . Another school of thought<br />
suggests decreased corneal sensation from contact<br />
lens wear leads to a ‘neurotrophic state’, which<br />
promotes inflammation and compromises the<br />
signal for tear production 10 .<br />
Lid wiper epitheliopathy (LWE) is a clinical sign that<br />
is gaining in popularity amongst practitioners when<br />
describing dry eye. LWE refers to disruption of the lid<br />
margin that ‘wipes’ across the anterior eye surface or<br />
contact lens during blinking (Fig 2.). Studies suggest<br />
BY ALEX PETTY*<br />
Get to the main<br />
cause of dry,<br />
irritated eyes*<br />
that the presence of LWE is highly associated with<br />
discomfort in contact lens wearers, occurring in<br />
80% of symptomatic contact lens patients versus<br />
13% for asymptomatic wearers 11 . LWE is a sign of<br />
increased friction with each blink; expected in a<br />
dry eye or with the use of a poorly wetting contact<br />
lens. Efron et al.’s recent comprehensive review<br />
of LWE states there is consistent evidence of a<br />
relation between contact lens surface friction and<br />
wearing comfort 12 .<br />
Improving dry eye symptoms in CL<br />
patients?<br />
The presence of LWE in CL patients has<br />
highlighted the importance of having a slippery<br />
lens surface to decrease friction and discomfort.<br />
Coles and Brenan showed that contact lenses<br />
with a higher lubricity tend to be more<br />
comfortable 13 . CL companies have been quick to<br />
release a number of excellent products that serve<br />
to increase the lubricity of patient’s lenses. These<br />
include Alcon’s Dailies Total1 water gradient<br />
lens and Bausch and Lomb’s “MoistureSeal”<br />
technology, incorporated in their Ultra range<br />
of lenses. We should not forget, however, that<br />
silicone hydrogels are naturally more hydrophobic<br />
than hydrogels due to their siloxane components,<br />
and may lead to decreased wettability, and<br />
therefore lubricity in certain wearers 14 . In<br />
these instances, hydrogel materials such as<br />
B+L’s Biotrue ONEday daily and Coopervision’s<br />
Proclear family (incorporating zwitterionic PC<br />
technology; still the only FDA material approved<br />
for patients that experience dryness with contact<br />
lens wear) may be useful. Alcon have also recently<br />
incorporated ‘Hydraglyde moisture matrix’,<br />
a hydrophilic compound that embeds onto a<br />
lens and decreases friction, into their hydrogen<br />
peroxide cleaning solution, AOSept, as well as their<br />
PureMoist multi-purpose disinfecting solution.<br />
Rigid lenses too can benefit from technologies<br />
to improve wettability. This includes the use of<br />
materials such as Optimum Extra with its very<br />
low wetting angles, and plasma-treatment of<br />
rigid lenses to decrease the hydrophobicity of<br />
4UP TO<br />
HOURS<br />
RELIEF 2<br />
CLINICALLY PROVEN<br />
the surface 15 . We should<br />
not forget to encourage all<br />
CL patients to use artificial<br />
tear drops as needed for an<br />
immediate improvement<br />
in lens lubricity, especially<br />
after longer-wear time.<br />
To avoid exacerbating any<br />
inflammatory aspects of<br />
dry eye, non-preserved<br />
formulations should be<br />
recommended.<br />
Options if the patient<br />
still cannot wear CLs<br />
comfortably?<br />
Despite advances in<br />
technology some patients,<br />
especially as they age and<br />
their tear volume naturally<br />
decreases, will continue<br />
to experience discomfort<br />
with soft contact lens wear.<br />
In this case sometimes no<br />
lens is better than any lens.<br />
Orthokeratology can be an<br />
excellent modality for patients<br />
who experience regular<br />
contact lens discomfort<br />
but otherwise only show<br />
mild signs of dry eye. One<br />
study showed that patients<br />
refitted from SiHy lenses<br />
into orthokeratology wear<br />
had a statistically significant<br />
increase in goblet cell density<br />
and improvement in dry eye<br />
symptoms after one month 16 .<br />
Anecdotally I have looked after<br />
a number of very satisfied<br />
orthokeratology patients that<br />
were previously unhappy with<br />
their SCL comfort.<br />
Scleral CLs<br />
A report on dry eye and<br />
contact lenses would not be<br />
complete without discussing<br />
scleral contact lenses. Sclerals<br />
are mainly reserved for<br />
patients with severe dry eye,<br />
such as Sjogren’s syndrome<br />
and graft-vs-host disease<br />
(GvHD), that do not find<br />
relief with other treatments.<br />
They are effective as the lens<br />
shields the eye and allows<br />
the post-lens fluid reservoir<br />
to bathe the compromised<br />
ocular surface during wear. Scleral lenses,<br />
including the PROSE lens (Prosthetic Replacement<br />
of the Ocular Surface Ecosystem; an impressive<br />
sounding scleral lens treatment that really just<br />
refers to onsite custom fitting at the B+L contact<br />
lens laboratory), have been shown to improve the<br />
visual function in patients with ocular surface<br />
disease over a five-year period 17 .<br />
A colleague of mine from the States, Dr<br />
Nate Schramm, a scleral lens expert from Fort<br />
Lauderdale, shared this relevant case with me<br />
recently: a 21-year-old man presented to his<br />
practice with severe dry eye symptoms. He has<br />
psoriasis and low testosterone and due to his<br />
programming occupation spent 75% of his day in<br />
front of a computer screen. He had been treated<br />
with meibomian gland probing and punctal<br />
cautery three months before and given a course<br />
of topical cyclosporin, however his symptoms did<br />
not improve. Examination showed instantaneous<br />
tear breakup, stagnant meibomian glands and<br />
Fig 1. Tear film structure with a soft contact lens<br />
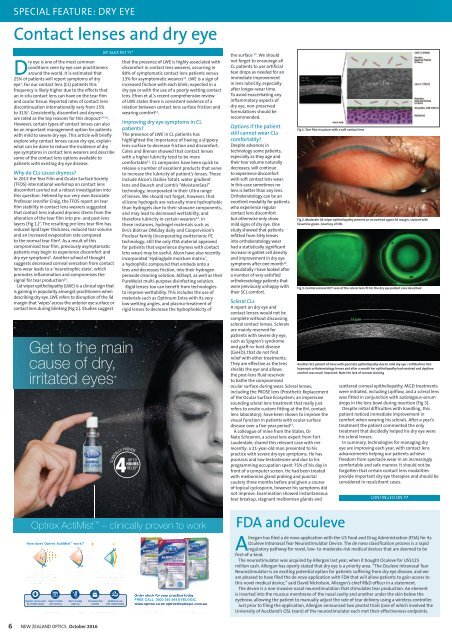
Fig 2. Moderate lid-wiper epitheliopathy present on an everted upper lid margin, stained with<br />
lissamine green. Courtesy of OSL<br />
Fig 3. Central corneal OCT scan of the scleral lens fit for the dry eye patient case described<br />
Another SCL patient of mine with punctate epitheliopathy due to mild dry eye. I refitted her into<br />
hyperopic orthokeratology lenses and after a month her epitheliopathy had resolved and daytime<br />
comfort was much improved. Note the lack of corneal staining<br />
scattered corneal epitheliopathy. MGD treatments<br />
were initiated, including Lipiflow, and a scleral lens<br />
was fitted in conjunction with autologous-serum<br />
drops in the lens bowl during insertion (Fig 3).<br />
Despite initial difficulties with handling, this<br />
patient noticed immediate improvement in<br />
comfort when wearing his sclerals. After a year’s<br />
treatment the patient commented the only<br />
treatment that decidedly helped his dry eye were<br />
his scleral lenses.<br />
In summary, technologies for managing dry<br />
eye are improving each year, with contact lens<br />
advancements helping our patients achieve<br />
freedom from spectacle wear in an increasingly<br />
comfortable and safe manner. It should not be<br />
forgotten that certain contact lens modalities<br />
provide important dry eye therapies and should be<br />
considered in recalcitrant cases.<br />
CONTINUED ON P7<br />
Optrex ActiMist – clinically proven to work<br />
How does Optrex ActiMist work?<br />
Optrex ActiMist contains<br />
liposomes (tiny bubbles fi lled with<br />
moisture) that migrate across the<br />
surface of the eyelid and collect<br />
at the edges of the eye.<br />
Hygienic. Can be used<br />
by multiple people<br />
Lasts 6 months<br />
after opening<br />
These liposomes mix with natural<br />
lipids on the eyelid.<br />
Won’t smudge<br />
make-up<br />
ActiMist liposomes<br />
ActiMist liposomes<br />
When the eyes are open the new<br />
lipid mixture spreads over the<br />
whole tear fi lm, and helps fi ll the<br />
gaps to restore the damaged<br />
lipid layer.<br />
Convenient and<br />
portable<br />
Natural lipids<br />
Suitable for use<br />
with contact lenses<br />
Order stock for your practice today<br />
FREE CALL 0800 393 564 EYELOGIC<br />
www.optrex.co.nz optrex@eyelogic.com.au<br />
Always read the label. Use only as directed. If symptoms persist, see your healthcare professional. †When wearing make-up, it is recommended to apply from 20cm. *Due to disturbed lipid layer of the tear fi lm. References:<br />
1. Lee S et al. Klin Monatsbl Augenheilkd 2004; 221:1–12. 2. Khaireddin R, Schmidt KG. Klin Monatsbl Augenheilkd. 2010; 227: 128-134. 3. Pult H et al. Contact Lens Anterior <strong>Eye</strong> 2012, 35:203-207. Reckitt Benckiser, Auckland. TAPS DA1541<br />
FDA and Oculeve<br />
Allergan has filed a de novo application with the US Food and Drug Administration (FDA) for its<br />
Oculeve Intranasal Tear Neurostimulator Device. The de novo classification process is a rapid<br />
regulatory pathway for novel, low- to-moderate-risk medical devices that are deemed to be<br />
first-of-a-kind.<br />
The neurostimulator was acquired by Allergan last year, when it bought Oculeve for US$125<br />
million cash. Allergan has openly stated that dry eye is a priority area. “The Oculeve Intranasal Tear<br />
Neurostimulator is an exciting potential option for patients suffering from dry eye disease, and we<br />
are pleased to have filed the de novo application with FDA that will allow patients to gain access to<br />
this novel medical device,” said David Nicholson, Allergon’s chief R&D officer in a statement.<br />
The device is a non-invasive nasal neurostimulation that stimulates tear production. An element<br />
is inserted into the mucous membrane of the nasal cavity and another under the skin below the<br />
eyebrow, allowing the patient to manually adjust the rate of tear delivery using a wireless controller.<br />
Just prior to filing the application, Allergan announced two pivotal trials (one of which involved the<br />
University of Auckland’s OSL team) of the neurostimulator each met their effectiveness endpoints.<br />
6 NEW ZEALAND OPTICS October <strong>2016</strong>