Chapter 21. Adina Paytan. Tracing the Sources and Biogeochemical Cycling of Phosphorus in Aquatic Systems Using Isotopes of Oxygen in Phosphate
Create successful ePaper yourself
Turn your PDF publications into a flip-book with our unique Google optimized e-Paper software.
21 <strong>Trac<strong>in</strong>g</strong> <strong>the</strong> <strong>Sources</strong> <strong>and</strong> <strong>Biogeochemical</strong> <strong>Cycl<strong>in</strong>g</strong> <strong>of</strong> <strong>Phosphorus</strong> 431<br />
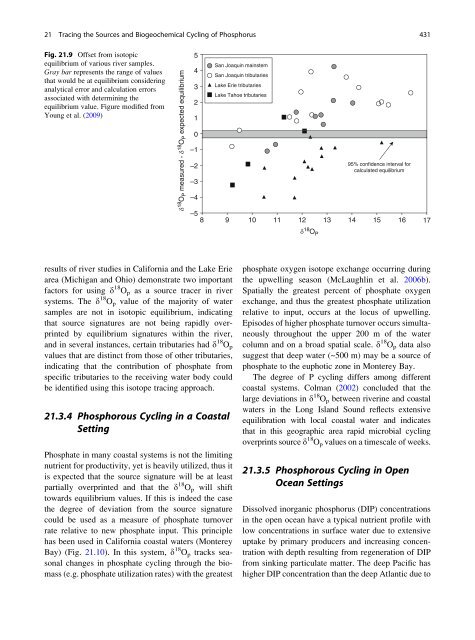
Fig. <strong>21.</strong>9 Offset from isotopic<br />
equilibrium <strong>of</strong> various river samples.<br />
Gray bar represents <strong>the</strong> range <strong>of</strong> values<br />
that would be at equilibrium consider<strong>in</strong>g<br />
analytical error <strong>and</strong> calculation errors<br />
associated with determ<strong>in</strong><strong>in</strong>g <strong>the</strong><br />
equilibrium value. Figure modified from<br />
Young et al. (2009)<br />
d 18 O P measured - d 18 O P expected equilibrium<br />
5<br />
4<br />
3<br />
2<br />
1<br />
0<br />
–1<br />
–2<br />
–3<br />
–4<br />
San Joaqu<strong>in</strong> ma<strong>in</strong>stem<br />
San Joaqu<strong>in</strong> tributaries<br />
Lake Erie tributaries<br />
Lake Tahoe tributaries<br />
95% confidence <strong>in</strong>terval for<br />
calculated equilibrium<br />
–5<br />
8 9 10 11 12 13 14 15<br />
d 18 O P<br />
16 17<br />
results <strong>of</strong> river studies <strong>in</strong> California <strong>and</strong> <strong>the</strong> Lake Erie<br />
area (Michigan <strong>and</strong> Ohio) demonstrate two important<br />
factors for us<strong>in</strong>g d 18 O p as a source tracer <strong>in</strong> river<br />
systems. The d 18 O p value <strong>of</strong> <strong>the</strong> majority <strong>of</strong> water<br />
samples are not <strong>in</strong> isotopic equilibrium, <strong>in</strong>dicat<strong>in</strong>g<br />
that source signatures are not be<strong>in</strong>g rapidly overpr<strong>in</strong>ted<br />
by equilibrium signatures with<strong>in</strong> <strong>the</strong> river,<br />
<strong>and</strong> <strong>in</strong> several <strong>in</strong>stances, certa<strong>in</strong> tributaries had d 18 O p<br />
values that are dist<strong>in</strong>ct from those <strong>of</strong> o<strong>the</strong>r tributaries,<br />
<strong>in</strong>dicat<strong>in</strong>g that <strong>the</strong> contribution <strong>of</strong> phosphate from<br />
specific tributaries to <strong>the</strong> receiv<strong>in</strong>g water body could<br />
be identified us<strong>in</strong>g this isotope trac<strong>in</strong>g approach.<br />
<strong>21.</strong>3.4 Phosphorous <strong>Cycl<strong>in</strong>g</strong> <strong>in</strong> a Coastal<br />
Sett<strong>in</strong>g<br />
<strong>Phosphate</strong> <strong>in</strong> many coastal systems is not <strong>the</strong> limit<strong>in</strong>g<br />
nutrient for productivity, yet is heavily utilized, thus it<br />
is expected that <strong>the</strong> source signature will be at least<br />
partially overpr<strong>in</strong>ted <strong>and</strong> that <strong>the</strong> d 18 O p will shift<br />
towards equilibrium values. If this is <strong>in</strong>deed <strong>the</strong> case<br />
<strong>the</strong> degree <strong>of</strong> deviation from <strong>the</strong> source signature<br />
could be used as a measure <strong>of</strong> phosphate turnover<br />
rate relative to new phosphate <strong>in</strong>put. This pr<strong>in</strong>ciple<br />
has been used <strong>in</strong> California coastal waters (Monterey<br />
Bay) (Fig. <strong>21.</strong>10). In this system, d 18 O p tracks seasonal<br />
changes <strong>in</strong> phosphate cycl<strong>in</strong>g through <strong>the</strong> biomass<br />
(e.g. phosphate utilization rates) with <strong>the</strong> greatest<br />
phosphate oxygen isotope exchange occurr<strong>in</strong>g dur<strong>in</strong>g<br />
<strong>the</strong> upwell<strong>in</strong>g season (McLaughl<strong>in</strong> et al. 2006b).<br />
Spatially <strong>the</strong> greatest percent <strong>of</strong> phosphate oxygen<br />
exchange, <strong>and</strong> thus <strong>the</strong> greatest phosphate utilization<br />
relative to <strong>in</strong>put, occurs at <strong>the</strong> locus <strong>of</strong> upwell<strong>in</strong>g.<br />
Episodes <strong>of</strong> higher phosphate turnover occurs simultaneously<br />
throughout <strong>the</strong> upper 200 m <strong>of</strong> <strong>the</strong> water<br />
column <strong>and</strong> on a broad spatial scale. d 18 O p data also<br />
suggest that deep water (~500 m) may be a source <strong>of</strong><br />
phosphate to <strong>the</strong> euphotic zone <strong>in</strong> Monterey Bay.<br />
The degree <strong>of</strong> P cycl<strong>in</strong>g differs among different<br />
coastal systems. Colman (2002) concluded that <strong>the</strong><br />
large deviations <strong>in</strong> d 18 O p between river<strong>in</strong>e <strong>and</strong> coastal<br />
waters <strong>in</strong> <strong>the</strong> Long Isl<strong>and</strong> Sound reflects extensive<br />
equilibration with local coastal water <strong>and</strong> <strong>in</strong>dicates<br />
that <strong>in</strong> this geographic area rapid microbial cycl<strong>in</strong>g<br />
overpr<strong>in</strong>ts source d 18 O p values on a timescale <strong>of</strong> weeks.<br />
<strong>21.</strong>3.5 Phosphorous <strong>Cycl<strong>in</strong>g</strong> <strong>in</strong> Open<br />
Ocean Sett<strong>in</strong>gs<br />
Dissolved <strong>in</strong>organic phosphorus (DIP) concentrations<br />
<strong>in</strong> <strong>the</strong> open ocean have a typical nutrient pr<strong>of</strong>ile with<br />
low concentrations <strong>in</strong> surface water due to extensive<br />
uptake by primary producers <strong>and</strong> <strong>in</strong>creas<strong>in</strong>g concentration<br />
with depth result<strong>in</strong>g from regeneration <strong>of</strong> DIP<br />
from s<strong>in</strong>k<strong>in</strong>g particulate matter. The deep Pacific has<br />
higher DIP concentration than <strong>the</strong> deep Atlantic due to