The Staphylococcus aureus secretome - TI Pharma
The Staphylococcus aureus secretome - TI Pharma
The Staphylococcus aureus secretome - TI Pharma
Create successful ePaper yourself
Turn your PDF publications into a flip-book with our unique Google optimized e-Paper software.
Chapter 3<br />
strain of a patient who suffered from a wound infection induced by this strain (isolate H) one<br />
year before. We found the hlb converting phage FaSa3 in all these isolates, except for isolate<br />
G, as might be expected from the phage dynamics during infection (Goerke et al., 2006;<br />
Goerke et al., 2004). In accordance with this, the phenotype of strain G288 changed from Hlb<br />
positive (G) to Hlb negative (C, D, H, I, J, K) (Table 3). However, the prophage did not<br />
significantly alter the expression of other virulence-associated genes (Figure 1). This might be<br />
mainly due to the fact that RNAIII was not expressed in these isolates under our experimental<br />
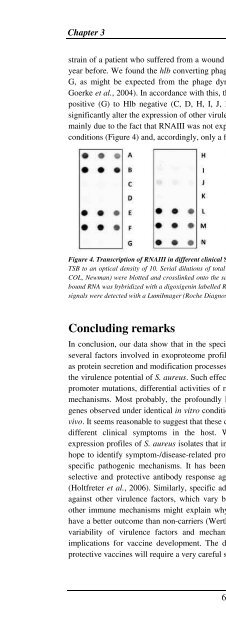
conditions (Figure 4) and, accordingly, only a few virulence factors were produced.<br />
Figure 4. Transcription of RNAIII in different clinical S. <strong>aureus</strong> isolates. RNA was prepared from cells grown in<br />
TSB to an optical density of 10. Serial dilutions of total RNA of clinical isolates and reference strains (RN6390,<br />
COL, Newman) were blotted and crosslinked onto the same positively charged nylon membrane. <strong>The</strong> membranebound<br />
RNA was hybridized with a digoxigenin labelled RNA probe complementary to RNAIII. Chemiluminescence<br />
signals were detected with a LumiImager (Roche Diagnostics, Mannheim, Germany).<br />
Concluding remarks<br />
In conclusion, our data show that in the species S. <strong>aureus</strong>, genome plasticity is only one of<br />
several factors involved in exoproteome profile heterogeneity. Expression regulation as well<br />
as protein secretion and modification processes add further dimensions to the heterogeneity of<br />
the virulence potential of S. <strong>aureus</strong>. Such effects might be further enhanced and fine-tuned by<br />
promoter mutations, differential activities of regulatory molecules and translation regulation<br />
mechanisms. Most probably, the profoundly heterogeneous expression pattern of virulence<br />
genes observed under identical in vitro conditions reflects a very high degree of variability in<br />
vivo. It seems reasonable to suggest that these different virulence protein patterns are linked to<br />
different clinical symptoms in the host. We are currently comparing virulence gene<br />
expression profiles of S. <strong>aureus</strong> isolates that induced very similar symptoms. In this way, we<br />
hope to identify symptom-/disease-related proteomic signatures that may help in elucidating<br />
specific pathogenic mechanisms. It has been shown that S. <strong>aureus</strong> carriers mount a very<br />
selective and protective antibody response against superantigens of their colonizing strains<br />
(Holtfreter et al., 2006). Similarly, specific adaptive immune responses might also be raised<br />
against other virulence factors, which vary between strains to a similar extent. <strong>The</strong>se and<br />
other immune mechanisms might explain why S. <strong>aureus</strong> carriers with bacteremia generally<br />
have a better outcome than non-carriers (Wertheim et al., 2004). Finally, given the extensive<br />
variability of virulence factors and mechanisms in S. <strong>aureus</strong>, our study has important<br />
implications for vaccine development. <strong>The</strong> data strongly suggest that the development of<br />
protective vaccines will require a very careful selection and combination of bacterial antigens.<br />
66