The Staphylococcus aureus secretome - TI Pharma
The Staphylococcus aureus secretome - TI Pharma
The Staphylococcus aureus secretome - TI Pharma
You also want an ePaper? Increase the reach of your titles
YUMPU automatically turns print PDFs into web optimized ePapers that Google loves.
Chapter 5<br />
incubated at 95ºC. Proteins were separated by SDS-PAGE using precast NuPage gels (Invitrogen) and<br />
subsequently blotted onto a nitrocellulose membrane (Protran ® , Schleicher & Schuell). <strong>The</strong> presence of<br />
IsaA, Aly or DsbA was monitored by immunodetection with specific polyclonal antibodies raised in<br />
mice (IsaA, Aly) or rabbits (DsbA (Kouwen et al., 2007)) at 1:10.000 dilution. Bound primary<br />
antibodies were visualized using fluorescent IgG secondary antibodies (IRDye 800 CW goat antimouse/anti-rabbit<br />
from LiCor Biosciences). Membranes were scanned for fluorescence at 800 nm using<br />
the Odyssey Infrared Imaging System (LiCor Biosciences).<br />
Results<br />
<strong>The</strong> exoproteomes of secG and secY2 mutant S. <strong>aureus</strong> strains<br />
To investigate the roles of SecG and SecY2 in the biogenesis of the S. <strong>aureus</strong> exoproteome,<br />
the respective genes were completely deleted from the chromosome of S. <strong>aureus</strong> strain<br />
RN4220. This resulted in the single mutant strains ∆secG and ∆secY2, and the double mutant<br />
∆secG ∆secY2. Next, cells of these mutants were grown in TSB medium until they reached<br />
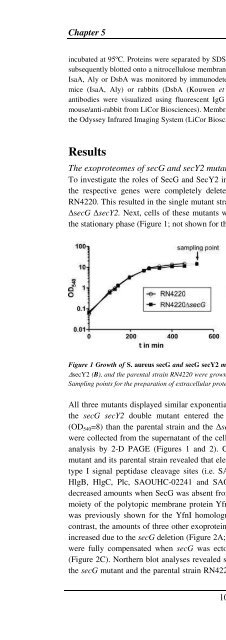
the stationary phase (Figure 1; not shown for the ∆secY2 strain).<br />
Figure 1 Growth of S. <strong>aureus</strong> secG and secG secY2 mutants. <strong>The</strong> S. <strong>aureus</strong> strains RN4220 ∆secG (A), ∆secG<br />
∆secY2 (B), and the parental strain RN4220 were grown in 100 ml TSB medium under vigorous shaking at 37 o C.<br />
Sampling points for the preparation of extracellular proteins are indicated in the growth curve by arrows.<br />
All three mutants displayed similar exponential growth rates as the parental strain. However,<br />
the secG secY2 double mutant entered the stationary phase at a lower optical density<br />
(OD540=8) than the parental strain and the ∆secG mutant (OD540=15). Extracellular proteins<br />
were collected from the supernatant of the cell cultures that had reached stationary phase for<br />
analysis by 2-D PAGE (Figures 1 and 2). Comparison of the exoproteomes of the secG<br />
mutant and its parental strain revealed that eleven proteins with Sec-type signal peptides and<br />
type I signal peptidase cleavage sites (i.e. SAOUHSC-00094, SdrD, Sle1, Geh, Hlb, HlY,<br />
HlgB, HlgC, Plc, SAOUHC-02241 and SAOUHSC-02979) were present in significantly<br />
decreased amounts when SecG was absent from the cells. This was also true for the secreted<br />
moiety of the polytopic membrane protein YfnI, which is processed by signal peptidase I as<br />
was previously shown for the YfnI homologue of B. subtilis (Antelmann et al., 2001). In<br />
contrast, the amounts of three other exoproteins (i.e. IsaA, SsaA, and Spa) were considerably<br />
increased due to the secG deletion (Figure 2A; Table 3A). <strong>The</strong>se effects of the secG mutation<br />
were fully compensated when secG was ectopically expressed from plasmid secG-pCN51<br />
(Figure 2C). Northern blot analyses revealed similar transcript levels for geh, hlb and spa in<br />
the secG mutant and the parental strain RN4220. This shows that the changes in the amounts<br />
100