The Staphylococcus aureus secretome - TI Pharma
The Staphylococcus aureus secretome - TI Pharma
The Staphylococcus aureus secretome - TI Pharma
Create successful ePaper yourself
Turn your PDF publications into a flip-book with our unique Google optimized e-Paper software.
Synthetic effects of secG and secY2 mutations on exoproteome biogenesis in<br />
<strong>Staphylococcus</strong> <strong>aureus</strong><br />
Impaired export of cell wall-bound Sbi in secG mutant cells<br />
Western blotting experiments were performed to investigate whether particular protein export<br />
defects of the secG and secY2 mutants had remained unnoticed in the proteomic analyses.<br />
<strong>The</strong>se analyses included secreted proteins in the growth medium, non-covalently cell wall<br />
attached and cellular proteins of S. <strong>aureus</strong> strains RN4220 and S. <strong>aureus</strong> SH1000.<br />
Furthermore, we used specific antibodies against membrane proteins, lipoproteins, cell wall<br />
proteins and exoproteins. For most tested proteins no differences were detectable between the<br />
secG and/or secY2 mutant strains and their parental strain. However, these analyses showed<br />
that a band of ~50 kDa, which was cross-reactive with all tested sera, had disappeared from<br />
the fraction of non-covalently bound cell wall proteins of the secG mutant. It is known that<br />
proteins, such as protein A (Sasso et al., 1991) and Sbi (Zhang et al., 1998) have IgG-binding<br />
properties. To investigate whether the missing band would relate to protein A or Sbi, protein<br />
fractions from a spa mutant, and a spa sbi double mutant, were included in the Western<br />
blotting analyses.<br />
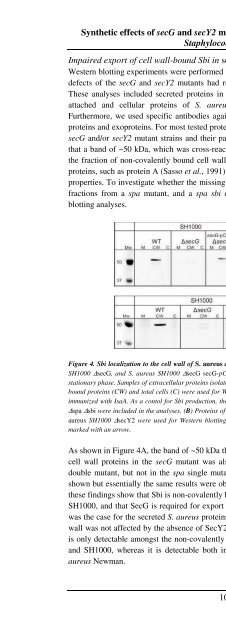
Figure 4. Sbi localization to the cell wall of S. <strong>aureus</strong> depends on SecG. (A) S. <strong>aureus</strong> SH1000 (WT), S. <strong>aureus</strong><br />
SH1000 ∆secG, and S. <strong>aureus</strong> SH1000 ∆secG secG-pCN51 were grown in TSB medium at 37 o C till the early<br />
stationary phase. Samples of extracellular proteins isolated from the growth medium (M), non-covalently cell wallbound<br />
proteins (CW) and total cells (C) were used for Western blotting and immunodetection with serum of mice<br />
immunized with IsaA. As a contol for Sbi production, the strains S. <strong>aureus</strong> Newman ∆spa and S. <strong>aureus</strong> Newman<br />
∆spa ∆sbi were included in the analyses. (B) Proteins of S. <strong>aureus</strong> SH1000 (wt), S. <strong>aureus</strong> SH1000 ∆secG, and S.<br />
<strong>aureus</strong> SH1000 ∆secY2 were used for Western blotting and immunodetection as in (A). <strong>The</strong> position of Sbi is<br />
marked with an arrow.<br />
As shown in Figure 4A, the band of ~50 kDa that was missing from the non-covalently bound<br />
cell wall proteins in the secG mutant was also missing from these proteins in the spa sbi<br />
double mutant, but not in the spa single mutant (only the results for S. <strong>aureus</strong> SH1000 are<br />
shown but essentially the same results were obtained for S. <strong>aureus</strong> RN4220). Taken together,<br />
these findings show that Sbi is non-covalently bound to the cell wall of S. <strong>aureus</strong> RN4220 and<br />
SH1000, and that SecG is required for export of Sbi from the cytoplasm to the cell wall. As<br />
was the case for the secreted S. <strong>aureus</strong> proteins detected by proteomics, Sbi export to the cell<br />
wall was not affected by the absence of SecY2 (Figure 4B). Finally, it is noteworthy that Sbi<br />
is only detectable amongst the non-covalently bound cell wall proteins of S. <strong>aureus</strong> RN4220<br />
and SH1000, whereas it is detectable both in a cell wall-bound and a secreted state in S.<br />
<strong>aureus</strong> Newman.<br />
105