Scarica n. 1/2011 - Società Italiana di Tabaccologia
Scarica n. 1/2011 - Società Italiana di Tabaccologia
Scarica n. 1/2011 - Società Italiana di Tabaccologia
Create successful ePaper yourself
Turn your PDF publications into a flip-book with our unique Google optimized e-Paper software.
26<br />
Original Article<br />
stimolazione dell’escrezione<br />
salivare. I campioni <strong>di</strong> saliva<br />
sono stati analizzati entro le<br />
due ore successive la raccolta<br />
a temperatura <strong>di</strong> 4 o C. I campioni<br />
sono stati preparati ed è<br />
stata analizzata la composizione<br />
cellulare degli immunociti<br />
presenti nella saliva utilizzando<br />
un citofluorometro a flusso<br />
BD FACSCanto II (Becton<br />
Dickinson, USA), secondo le<br />
procedure certificate, Patenta<br />
No. 2008120724 as of 23.05.08<br />
(Teplova S.N., Kochengina S.A.,<br />
et al.). La procedura della preparazione<br />
dei campione è stata<br />
basata sulla tecnologia del risciacquo<br />
on con RPMI e bicarbonato.<br />
La composizione delle<br />
cellule immunitarie nella saliva<br />
è stata analizzata utilizzando i<br />
kit basati su anticorpi monoclonali<br />
(MAK) caratterizzati da<br />
quattro coloranti fluorescenti,<br />
la Fluoresceina isotiocianato (FITC), la Ficoeritrina (PE),<br />
la Proteina Peri<strong>di</strong>na-Clorofilla (PerCP), l’Alloficocianina<br />
(APC) dai MultiTest (fabbricati da Beston Dickinson, USA)<br />
e un colorante vitale, il 7-AAD. L’analisi citofluorometrica<br />
della composizione cellulare della saliva ha in<strong>di</strong>viduato<br />
almeno 10,000 eventi. Il numero delle cellule vitali che<br />
esprimevano un antigene leucocitario comune, CD45+,<br />
incluse le popolazioni <strong>di</strong> immunociti come i granulociti<br />
(CD45+CD13+CD14-), i monociti (CD45+CD14+CD13–),<br />
i linfociti T citotossici (CD45+CD3+CD8+), i T-helpers<br />
(CD45+CD3+CD4+), le cellule NK (CD45+CD56+16+), è<br />
stato rilevato usando il 7-AAD. Le cellule sono state <strong>di</strong>stinte<br />
in base all’espressione <strong>di</strong> un marker <strong>di</strong> <strong>di</strong>fferenziazione,<br />
il CD45. Il rapporto CD45+/CD45- delle cellule e<br />
dei pellet cellulari presenti nella saliva sono illustrati nella<br />
Figura 1. Le cellule vitali con un marker <strong>di</strong> <strong>di</strong>fferenziazione<br />
lineare, CD45+, è mostrato nella Figura 2. I livelli<br />
<strong>di</strong> IL-17 nella saliva sono stati misurati utilizzando l’immunoassay<br />
enzimatico con un sistema <strong>di</strong> test, il Human<br />
IL-17A ELISA BMS2017 e BMS2017TEN (Bender MedSystems,<br />
Vienna, Austria), per le analisi dei flui<strong>di</strong> biologici,<br />
con un fotometro, Multiscan plus (Labsystems, Finlanda),<br />
450 nm. La sensitività del test-system era <strong>di</strong> 1 pg/mL. Sono<br />
stati misurati nella saliva i livelli <strong>di</strong> IL-17 prodotta da una<br />
relativamente nuova linea <strong>di</strong> cellule T, Th17, correlati ai<br />
linfociti CD+ [8]. Lo stu<strong>di</strong>o è stato approvato dalla commisione<br />
etica del Dipartimento Sanitario Centrale No.71<br />
dell’FMBA Russia. Il consenso informato è stato ottenuto<br />
da ogni partecipante e lo stu<strong>di</strong>o è stato eseguito secondo<br />
la Dichiarazione <strong>di</strong> Helsinki sui Principi Etici per la Ricerca<br />
Me<strong>di</strong>ca che Coinvolge Soggetti Umani.<br />
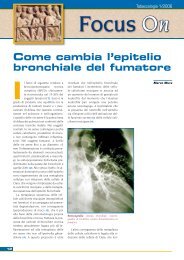
Bronco normale in non fumatore<br />
BPCO in fumatore<br />
Altynbaeva E I et al, <strong>Tabaccologia</strong> <strong>2011</strong>; 1: 23-29<br />
water, in dry glass flasks without<br />
stimulation of salivary <strong>di</strong>scharge.<br />
Saliva specimens were analyzed<br />
within 2 hrs after collection at the<br />
maintained at 40C. Saliva specimens<br />
were prepared and cellular<br />
composition of immunocytes in<br />
saliva was analyzed using a flow<br />
cytofluorometer, BD FACSCanto II<br />
(Becton Dickinson, USA), in accordance<br />
with the certified procedure,<br />
Patent No. 2008120724<br />
as of 23.05.08 (Teplova S.N., Kochengina<br />
S.A., et al.).<br />
The procedure of samples preparation<br />
was based on the rinse<br />
technology with RPMI and bicarbonate.<br />
The population composition<br />
of immune cells in saliva<br />
was analyzed using the monoclonal<br />
antibody-based kits (MAK)<br />
labeled with four fluorescent<br />
dyes, Fluorescein Isothiocyanate<br />
(FITC), Phycoerythrin (PE), Peri<strong>di</strong>n-Chlorophyll<br />
Protein (PerCP),<br />
Allophycocyanin (APC) from the MultiTest series (manufactured<br />
by Becton Dickinson, USA), and a vital dye,<br />
7-AAD.<br />
The cytofluorometric analysis of the cellular composition<br />
of saliva accounted for at least 10,000 events. The<br />
number of viable cells expressing a leukocyte common<br />
antigen, CD45+, was accounted for using 7-AAD, inclu<strong>di</strong>ng<br />
populations of immunocytes such as granulocytes<br />
(CD45+CD13+CD14-), monocytes (CD45+CD14+CD13–),<br />
T-cytotoxic lymphocytes (CD45+CD3+CD8+), T-helpers<br />
(CD45+CD3+CD4+), NK-cells (CD45+CD56+16+).<br />
Cells were separated based on the expression of a <strong>di</strong>fferentiation<br />
marker, CD45. CD45+/CD45- ratio of cells and<br />
cell pellets in saliva is illustrated in Figure 1. The gate of<br />
viable cells with a linear <strong>di</strong>fferentiation marker, CD45+, is<br />
shown on Figure 2.<br />
Levels of IL-17 in saliva were measured using enzyme<br />
immunoassay with a test-system, Human IL-17A ELISA<br />
BMS2017 and BMS2017TEN (Bender MedSystems, Vienna,<br />
Austria), for analyses of biological fluids, with a plate<br />
photometer, Multiscan plus (Labsystems, Finland), 450<br />
nm. The test-system sensitivity was 1 pg/mL. The levels<br />
of IL-17 produced by a relatively new line of T-cells, Th17,<br />
related to CD+ lymphocytes, were measured in saliva [8] [8].<br />
The study was approved by the Ethical Committee of<br />
the Central Me<strong>di</strong>cal Sanitary Department No. 71 of the<br />
FMBA Russia.<br />
The informed consent was obtained from each participant,<br />
and the study was performed in accordance with the<br />
Declaration of Helsinki on Ethical Principles for Me<strong>di</strong>cal<br />
Research Involving Human Subjects.