You also want an ePaper? Increase the reach of your titles
YUMPU automatically turns print PDFs into web optimized ePapers that Google loves.
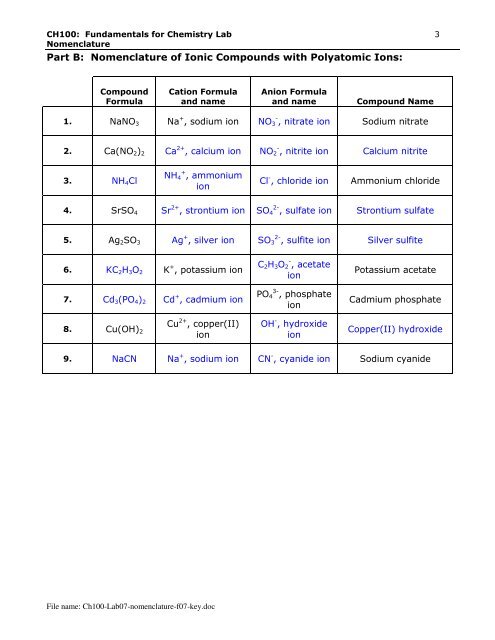
CH100: Fundamentals for Chemistry Lab 3<br />
Nomenclature<br />
Part B: Nomenclature of Ionic Compounds with Polyatomic Ions:<br />
Compound<br />
Formula<br />
Cation Formula<br />
and name<br />
Anion Formula<br />
and name<br />
Compound Name<br />
1. NaNO 3 Na + , sodium ion NO 3 - , nitrate ion Sodium nitrate<br />
2. Ca(NO 2 ) 2 Ca 2+ , calcium ion NO 2 - , nitrite ion Calcium nitrite<br />
3. NH 4 Cl<br />
NH 4 + , ammonium<br />
ion<br />
Cl - , chloride ion<br />
Ammonium chloride<br />
4. SrSO 4 Sr 2+ , strontium ion SO 4 2- , sulfate ion Strontium sulfate<br />
5. Ag 2 SO 3 Ag + , silver ion SO 3 2- , sulfite ion Silver sulfite<br />
6. KC 2 H 3 O 2 K + , potassium ion<br />
7. Cd 3 (PO 4 ) 2 Cd + , cadmium ion<br />
C 2 H 3 O 2 - , acetate<br />
ion<br />
PO 4 3- , phosphate<br />
ion<br />
Potassium acetate<br />
Cadmium phosphate<br />
8. Cu(OH) 2<br />
Cu 2+ , copper(II)<br />
ion<br />
OH - , hydroxide<br />
ion<br />
Copper(II) hydroxide<br />
9. NaCN Na + , sodium ion CN - , cyanide ion Sodium cyanide<br />
File name: <strong>Ch100</strong>-Lab07-<strong>nomenclature</strong>-<strong>f07</strong>-<strong>key</strong>.doc