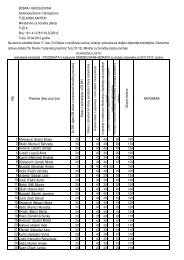
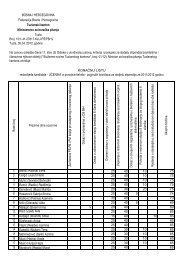
POZITIVNA LISTA LIJEKOVA TUZLANSKOG KANTONA A – lista ...
POZITIVNA LISTA LIJEKOVA TUZLANSKOG KANTONA A – lista ...
POZITIVNA LISTA LIJEKOVA TUZLANSKOG KANTONA A – lista ...
You also want an ePaper? Increase the reach of your titles
YUMPU automatically turns print PDFs into web optimized ePapers that Google loves.
Redni<br />
broj<br />
Šifra ATC<br />
Nezaštićeno ime<br />
(generičko ime) INN<br />
Zaštićeno ime Proizvođač Oblik Jačina Pakovanje<br />
Cijena<br />
pakovanj<br />
a sa PDVom<br />
(KM)<br />
Režim<br />
propisivanja<br />
1 2 3 4 5 7 8 9 10 1110<br />
271 C09BA03 lizinopril, hidrohlorotiazid LOPRIL H plus BOSNALIJEK d.d. tableta<br />
20 mg + 25<br />
mg<br />
20 tableta 10,65 Rp<br />
272 C09BA03 lizinopril, hidrohlorotiazid HYPERIL Plus ZADA Pharmaceuticals d.o.o. tableta<br />
20 mg + 25<br />
mg<br />
30 tableta 15,95 Rp<br />
273 C09BA03 lizinopril, hidrohlorotiazid IRUZID FARMAVITA d.o.o. Sarajevo tableta<br />
20 mg + 25<br />
mg<br />
30 tableta 15,95 Rp<br />
274 C09BA03 lizinopril, hidrohlorotiazid LOPRIL H plus BOSNALIJEK d.d. tableta<br />
20 mg + 25<br />
mg<br />
30 tableta 15,95 Rp<br />
275 C09BA03 lizinopril, hidrohlorotiazid LISINOCOMP Genericon GENERICON PHARMA GmbH tableta<br />
20 mg + 25<br />
mg<br />
30 tableta 15,95 Rp<br />
276 C09CA03 valsartan ATENZIO ZADA Pharmaceuticals d.o.o. tableta 160 mg<br />
30 film<br />
tableta<br />
24,8 Rp/specE<br />
277 C09CA03 valsartan VAL FARMAVITA d.o.o. Sarajevo tableta 160 mg 28 tableta 23,15 Rp/specE<br />
278 C09CA03 valsartan VALSACOR KRKA, tovarna zdravil, d.d. tableta 160 mg 28 tableta 23,15 Rp/specE<br />
279 C09CA03 valsartan VAL FARMAVITA d.o.o. Sarajevo tableta 80 mg 28 tableta 17,8 Rp/specE<br />
280 C09CA03 valsartan VALSACOR KRKA, tovarna zdravil, d.d. tableta 80 mg 28 tableta 17,8 Rp/specE<br />
281 C09CA03 valsartan ATENZIO ZADA Pharmaceuticals d.o.o. tableta 80 mg<br />
30 film<br />
tableta<br />
19,05 Rp/specE<br />
282 C09DA03 valsartan /HCTZ CO-DIOVAN<br />
NOVARTIS PHARMA Services<br />
AG<br />
tableta 160/12,5 mg 28 tableta 28,35 Rp/specE<br />
283 C09DA03 valsartan /HCTZ VAL Plus FARMAVITA d.o.o. Sarajevo tableta 160/12,5 mg 28 tableta 28,35 Rp/specE<br />
284 C09DA03 valsartan /HCTZ VALSACOMBI KRKA, tovarna zdravil, d.d. tableta 160/12,5 mg 28 tableta 28,35 Rp/specE<br />
285 C09DA03 valsartan /HCTZ ATENZIO PLUS ZADA Pharmaceuticals d.o.o. tableta 160/12,5 mg<br />
30 film<br />
tableta<br />
30,4 Rp/specE<br />
286 C09DA03 valsartan /HCTZ CO-DIOVAN<br />
NOVARTIS PHARMA Services<br />
AG<br />
tableta 80/12,5 mg 28 tableta 23,8 Rp/specE<br />
287 C09DA03 valsartan /HCTZ VAL Plus FARMAVITA d.o.o. Sarajevo tableta 80/12,5 mg 28 tableta 23,8 Rp/specE<br />
288 C09DA03 valsartan /HCTZ VALSACOMBI KRKA, tovarna zdravil, d.d. tableta 80/12,5 mg 28 tableta 23,8 Rp/specE<br />
289 C09DA03 valsartan /HCTZ ATENZIO PLUS ZADA Pharmaceuticals d.o.o. tableta 80/12,5 mg<br />
30 film<br />
tableta<br />
25,5 Rp/specE<br />
290 C10AA01 simvastatin PROTECTA FARMAVITA d.o.o. Sarajevo tableta 10 mg 28 tableta 9,15 Rp/ F<br />
291 C10AA01 simvastatin VASILIP KRKA, tovarna zdravil, d.d. tableta 10 mg 28 tableta 9,15 Rp/ F<br />
292 C10AA01 simvastatin CHOLIPAM HEMOFARM tableta 10 mg 20 tableta 6,55 Rp/ F<br />
293 C10AA01 simvastatin HOLLESTA ALKALOID AD tableta 10 mg<br />
30 film<br />
tableta<br />
9,8 Rp/ F<br />
294 C10AA01 simvastatin HOLLESTA ALKALOID AD tableta 20 mg<br />
30 film<br />
tableta<br />
12,85 Rp/ F<br />
295 C10AA01 simvastatin PROTECTA FARMAVITA d.o.o. Sarajevo tableta 20 mg 28 tableta 12 Rp/ F<br />
296 C10AA01 simvastatin VASILIP KRKA, tovarna zdravil, d.d. tableta 20 mg 28 tableta 12 Rp/ F<br />
Rp/specE samo za bolesnike koji ne podnose ACE inhibitore, po preporucu specijalsita internista<br />
Rp/ F u primarnoj prevenciji u bolesnika kojem je nakon tromjeseènog pridržavanja dijete vrijednost ukupnog holesterola izna 7 mmol/L<br />
11