Unit 5 Naming Review
Unit 5 Naming Review
Unit 5 Naming Review
Create successful ePaper yourself
Turn your PDF publications into a flip-book with our unique Google optimized e-Paper software.
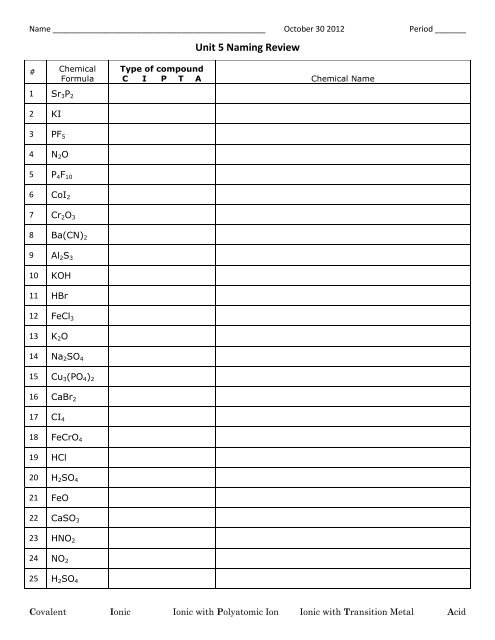
Name ________________________________________________ October 30 2012 Period _______<br />
<strong>Unit</strong> 5 <strong>Naming</strong> <strong>Review</strong><br />
# Chemical<br />
Formula<br />
Type of compound<br />
C I P T A Chemical Name<br />
1 Sr 3 P 2<br />
2 KI<br />
3 PF 5<br />
4 N 2 O<br />
5 P 4 F 10<br />
6 CoI 2<br />
7 Cr 2 O 3<br />
8 Ba(CN) 2<br />
9 Al 2 S 3<br />
10 KOH<br />
11 HBr<br />
12 FeCl 3<br />
13 K 2 O<br />
14 Na 2 SO 4<br />
15 Cu 3 (PO 4 ) 2<br />
16 CaBr 2<br />
17 CI 4<br />
18 FeCrO 4<br />
19 HCl<br />
20 H 2 SO 4<br />
21 FeO<br />
22 CaSO 3<br />
23 HNO 2<br />
24 NO 2<br />
25 H 2 SO 4<br />
Covalent Ionic Ionic with Polyatomic Ion Ionic with Transition Metal Acid
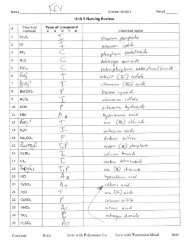
# Chemical Name C I P T A Cation Anion Chemical Formula<br />
1 carbonic acid A (ternary) H +1 CO 3 -2 H 2 CO 3<br />
2 rubidium selenide<br />
3 tin (II) chloride<br />
4 calcium phosphate<br />
5 lead (II) nitrite<br />
6 hydrobromic acid<br />
7 bromine pentafluoride C None None BF 5<br />
8 tetraarsenic decoxide<br />
9 magnesium iodide<br />
10 gold (I) chloride<br />
11 strontium fluoride<br />
12 hydroselenic acid<br />
13 cesium selenide<br />
14 nitric acid<br />
15 phosphorous trichloride<br />
16 carbon tetrachloride<br />
17 magnesium perchlorate<br />
18 lead (IV) iodide<br />
19 lithium chlorate<br />
20 potassium acetate<br />
21 tin (IV) oxide<br />
22 tricarbon octafluoride<br />
23 rubidium bromide<br />
24 chromic acid<br />
25 calcium nitrate<br />
Covalent Ionic Ionic with Polyatomic Ion Ionic with Transition Metal Acid