12.68_Notification_of_Registration_Sep12_v1(pdf) - Medicines ...
12.68_Notification_of_Registration_Sep12_v1(pdf) - Medicines ...
12.68_Notification_of_Registration_Sep12_v1(pdf) - Medicines ...
You also want an ePaper? Increase the reach of your titles
YUMPU automatically turns print PDFs into web optimized ePapers that Google loves.
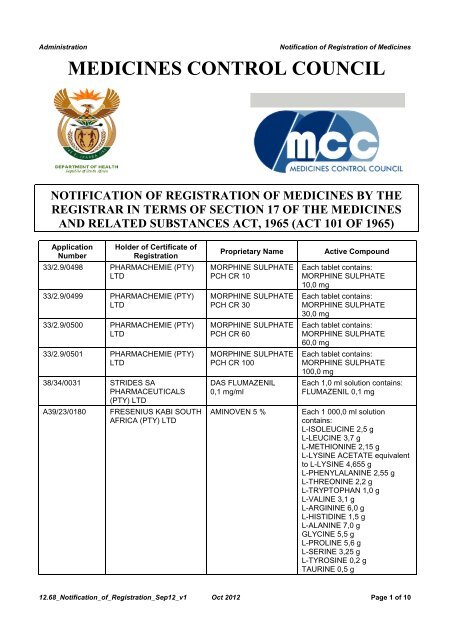
Administration <strong>Notification</strong> <strong>of</strong> <strong>Registration</strong> <strong>of</strong> <strong>Medicines</strong><br />
MEDICINES CONTROL COUNCIL<br />
NOTIFICATION OF REGISTRATION OF MEDICINES BY THE<br />
REGISTRAR IN TERMS OF SECTION 17 OF THE MEDICINES<br />
AND RELATED SUBSTANCES ACT, 1965 (ACT 101 OF 1965)<br />
Application<br />
Number<br />
Holder <strong>of</strong> Certificate <strong>of</strong><br />
<strong>Registration</strong><br />
33/2.9/0498 PHARMACHEMIE (PTY)<br />
LTD<br />
33/2.9/0499 PHARMACHEMIE (PTY)<br />
LTD<br />
33/2.9/0500 PHARMACHEMIE (PTY)<br />
LTD<br />
33/2.9/0501 PHARMACHEMIE (PTY)<br />
LTD<br />
38/34/0031 STRIDES SA<br />
PHARMACEUTICALS<br />
(PTY) LTD<br />
A39/23/0180 FRESENIUS KABI SOUTH<br />
AFRICA (PTY) LTD<br />
Proprietary Name Active Compound<br />
MORPHINE SULPHATE<br />
PCH CR 10<br />
MORPHINE SULPHATE<br />
PCH CR 30<br />
MORPHINE SULPHATE<br />
PCH CR 60<br />
MORPHINE SULPHATE<br />
PCH CR 100<br />
DAS FLUMAZENIL<br />
0,1 mg/ml<br />
Each tablet contains:<br />
MORPHINE SULPHATE<br />
10,0 mg<br />
Each tablet contains:<br />
MORPHINE SULPHATE<br />
30,0 mg<br />
Each tablet contains:<br />
MORPHINE SULPHATE<br />
60,0 mg<br />
Each tablet contains:<br />
MORPHINE SULPHATE<br />
100,0 mg<br />
Each 1,0 ml solution contains:<br />
FLUMAZENIL 0,1 mg<br />
AMINOVEN 5 % Each 1 000,0 ml solution<br />
contains:<br />
L-ISOLEUCINE 2,5 g<br />
L-LEUCINE 3,7 g<br />
L-METHIONINE 2,15 g<br />
L-LYSINE ACETATE equivalent<br />
to L-LYSINE 4,655 g<br />
L-PHENYLALANINE 2,55 g<br />
L-THREONINE 2,2 g<br />
L-TRYPTOPHAN 1,0 g<br />
L-VALINE 3,1 g<br />
L-ARGININE 6,0 g<br />
L-HISTIDINE 1,5 g<br />
L-ALANINE 7,0 g<br />
GLYCINE 5,5 g<br />
L-PROLINE 5,6 g<br />
L-SERINE 3,25 g<br />
L-TYROSINE 0,2 g<br />
TAURINE 0,5 g<br />
<strong>12.68</strong>_<strong>Notification</strong>_<strong>of</strong>_<strong>Registration</strong>_<strong>Sep12</strong>_<strong>v1</strong> Oct 2012 Page 1 <strong>of</strong> 10
Administration <strong>Notification</strong> <strong>of</strong> <strong>Registration</strong> <strong>of</strong> <strong>Medicines</strong><br />
Application<br />
Number<br />
Holder <strong>of</strong> Certificate <strong>of</strong><br />
<strong>Registration</strong><br />
A39/23/0181 FRESENIUS KABI SOUTH<br />
AFRICA (PTY) LTD<br />
A39/23/0183 FRESENIUS KABI SOUTH<br />
AFRICA (PTY) LTD<br />
A40/20.2.2/0437 ZYDUS HEALTHCARE SA<br />
(PTY) LTD<br />
A40/20.2.2/0438 ZYDUS HEALTHCARE SA<br />
(PTY) LTD<br />
Proprietary Name Active Compound<br />
AMINOVEN 10 % Each 1 000,0 ml solution<br />
contains:<br />
L-ISOLEUCINE 5,0 g<br />
L-LEUCINE 7,4 g<br />
L-METHIONINE 4,3 g<br />
L-LYSINE ACETATE equivalent<br />
to L-LYSINE 9,31 g<br />
L-PHENYLALANINE 5,1 g<br />
L-THREONINE 4,4 g<br />
L-TRYPTOPHAN 2,0 g<br />
L-VALINE 6,2 g<br />
L-ARGININE 12,0 g<br />
L-HISTIDINE 3,0 g<br />
L-ALANINE 14,0 g<br />
GLYCINE 11,0 g<br />
L-PROLINE 11,2 g<br />
L-SERINE 6,5 g<br />
L-TYROSINE 0,4 g<br />
TAURINE 1,0 g<br />
AMINOVEN 15 % Each 1 000,0 ml solution<br />
contains:<br />
L-ISOLEUCINE 5,2 g<br />
L-LEUCINE 8,9 g<br />
L-METHIONINE 3,8 g<br />
L-LYSINE ACETATE equivalent<br />
to L-LYSINE 15,655 g<br />
L-PHENYLALANINE 5,5 g<br />
L-THREONINE 8,6 g<br />
L-TRYPTOPHAN 1,6 g<br />
L-VALINE 5,5 g<br />
L-ARGININE 20,0 g<br />
L-HISTIDINE 7,3 g<br />
L-ALANINE 25,0 g<br />
GLYCINE 18,5 g<br />
L-PROLINE 17,0 g<br />
L-SERINE 9,6 g<br />
L-TYROSINE 0,4 g<br />
TAURINE 2,0 g<br />
ZYDUS<br />
FLUCONAZOLE<br />
150 mg<br />
ZYDUS<br />
FLUCONAZOLE<br />
200 mg<br />
Each capsule contains:<br />
FLUCONAZOLE 150,0 mg<br />
Each capsule contains:<br />
FLUCONAZOLE 200,0 mg<br />
41/21.2/0354 MSD (PTY) LTD JANUVIA 25 Each tablet contains:<br />
SITAGLIPTIN PHOSPATE<br />
equivalent to<br />
SITAGLIPTIN 25,0 mg<br />
41/21.2/0355 MSD (PTY) LTD JANUVIA 50 Each tablet contains:<br />
SITAGLIPTIN PHOSPATE<br />
equivalent to<br />
SITAGLIPTIN 50,0 mg<br />
<strong>12.68</strong>_<strong>Notification</strong>_<strong>of</strong>_<strong>Registration</strong>_<strong>Sep12</strong>_<strong>v1</strong> Oct 2012 Page 2 <strong>of</strong> 10
Administration <strong>Notification</strong> <strong>of</strong> <strong>Registration</strong> <strong>of</strong> <strong>Medicines</strong><br />
Application<br />
Number<br />
Holder <strong>of</strong> Certificate <strong>of</strong><br />
<strong>Registration</strong><br />
Proprietary Name Active Compound<br />
41/21.2/0356 MSD (PTY) LTD JANUVIA 100 Each tablet contains:<br />
SITAGLIPTIN PHOSPATE<br />
equivalent to<br />
SITAGLIPTIN 100,0 mg<br />
42/11.10/0263 RECKITT BENCKISER<br />
PHARMACEUTICALS<br />
(PTY) LTD<br />
42/26/0327 ACCORD HEALTHCARE<br />
(PTY) LTD<br />
42/26/0328 ACCORD HEALTHCARE<br />
(PTY) LTD<br />
42/26/0329 ACCORD HEALTHCARE<br />
(PTY) LTD<br />
GAVISCON PLUS<br />
TABLETS<br />
ACCORD MIDAZOLAM<br />
15 mg/3 ml<br />
ACCORD MIDAZOLAM<br />
50 mg/10 ml<br />
ACCORD MIDAZOLAM<br />
5 mg/5 ml<br />
Each tablet contains:<br />
SODIUM ALGINATE 250,0 mg<br />
SODIUM BICARBONATE<br />
106,5 mg<br />
CALCIUM CARBONATE<br />
187,5 mg<br />
Each 3,0 ml solution contains:<br />
MIDAZOLAM 15,0 mg<br />
Each 10,0 ml solution contains:<br />
MIDAZOLAM 50,0 mg<br />
Each 5,0 ml solution contains:<br />
MIDAZOLAM 5,0 mg<br />
42/11.5/0408 NORGINE (PTY) LTD MACROLYTE Each sachet <strong>of</strong> powder contains:<br />
POLYETHYLENE GLYCOL<br />
3 350 13,125 g<br />
SODIUM BICARBONATE<br />
178,5 mg<br />
SODIUM CHLORIDE 350,07 mg<br />
POTASSIUM CHLORIDE<br />
46,6 mg<br />
42/11.5/0409 NORGINE (PTY) LTD ISOPEG Each sachet <strong>of</strong> powder contains:<br />
POLYETHYLENE GLYCOL<br />
3 350 13,125 g<br />
SODIUM BICARBONATE<br />
178,5 mg<br />
SODIUM CHLORIDE 350,07 mg<br />
POTASSIUM CHLORIDE<br />
46,6 mg<br />
42/20.2/0567 BIOTECH<br />
LABORATORIES (PTY)<br />
LTD<br />
42/5.2/0847 ZYDUS HEALTHCARE SA<br />
(PTY) LTD<br />
42/5.2/0848 ZYDUS HEALTHCARE SA<br />
(PTY) LTD<br />
BIO METRONIDAZOLE<br />
IV<br />
BISOPROLOL ZYDUS 5<br />
TABLETS<br />
BISOPROLOL ZYDUS<br />
10 TABLETS<br />
42/20.1.1/1002 PHARMACARE LIMITED ASPEN<br />
LEVOFLOXACIN<br />
5 mg/ml<br />
42/26/1065 ACCORD HEALTHCARE<br />
(PTY) LTD<br />
42/26/1066 ACCORD HEALTHCARE<br />
(PTY) LTD<br />
42/7.1.5/1070 PHARMA DYNAMICS<br />
(PTY) LTD<br />
ACCORD<br />
OXALIPLATIN 100<br />
INTAS OXALIPLATIN<br />
100<br />
Each 100,0 ml solution contains:<br />
METRONIDAZOLE 500,0 mg<br />
Each tablet contains:<br />
BISOPROLOL FUMARATE<br />
5,0 mg<br />
Each tablet contains:<br />
BISOPROLOL FUMARATE<br />
10,0 mg<br />
Each 1,0 ml contains:<br />
LEVOFLOXACIN<br />
HEMIHYDRATE 5,0 mg<br />
Each vial contains:<br />
OXALIPLATIN 100,0 mg<br />
Each vial contains:<br />
OXALIPLATIN 100,0 mg<br />
DYNAFIL 25 mg Each tablet contains:<br />
SILDENAFIL CITRATE<br />
equivalent to<br />
SILDENAFIL 25,0 mg<br />
<strong>12.68</strong>_<strong>Notification</strong>_<strong>of</strong>_<strong>Registration</strong>_<strong>Sep12</strong>_<strong>v1</strong> Oct 2012 Page 3 <strong>of</strong> 10
Administration <strong>Notification</strong> <strong>of</strong> <strong>Registration</strong> <strong>of</strong> <strong>Medicines</strong><br />
Application<br />
Number<br />
Holder <strong>of</strong> Certificate <strong>of</strong><br />
<strong>Registration</strong><br />
42/7.1.5/1071 PHARMA DYNAMICS<br />
(PTY) LTD<br />
42/7.1.5/1072 PHARMA DYNAMICS<br />
(PTY) LTD<br />
Proprietary Name Active Compound<br />
DYNAFIL 50 mg Each tablet contains:<br />
SILDENAFIL CITRATE<br />
equivalent to<br />
SILDENAFIL 50,0 mg<br />
DYNAFIL 100 mg Each tablet contains:<br />
SILDENAFIL CITRATE<br />
equivalent to<br />
SILDENAFIL 100,0 mg<br />
42/21.2/1089 MSD (PTY) LTD JANUMET 50/500 Each tablet contains:<br />
SITAGLIPTIN PHOSPHATE<br />
equivalent to<br />
SITAGLIPTIN 50,0 mg<br />
METFORMIN<br />
HYDROCHLORIDE 500,0 mg<br />
42/21.2/1090 MSD (PTY) LTD JANUMET 50/850 Each tablet contains:<br />
SITAGLIPTIN PHOSPHATE<br />
equivalent to<br />
SITAGLIPTIN 50,0 mg<br />
METFORMIN<br />
HYDROCHLORIDE 850,0 mg<br />
42/21.2/1091 MSD (PTY) LTD JANUMET 50/1 000 Each tablet contains:<br />
SITAGLIPTIN PHOSPHATE<br />
equivalent to<br />
SITAGLIPTIN 50,0 mg<br />
METFORMIN<br />
HYDROCHLORIDE 1 000,0 mg<br />
42/20.1.1/1098 STRIDES SA<br />
PHARMACEUTICALS<br />
(PTY ) LTD<br />
STRIDES<br />
AZITHROMYCIN<br />
500 mg<br />
43/21.12/0010 MEDIVISION (PTY) LTD MEDIVISION<br />
ANASTROZOLE 1<br />
43/7.1.3/0059 ZYDUS HEALTHCARE SA<br />
(PTY) LTD<br />
43/7.1.3/0060 ZYDUS HEALTHCARE SA<br />
(PTY) LTD<br />
43/7.1.3/0061 ZYDUS HEALTHCARE SA<br />
(PTY) LTD<br />
43/20.1.1/0245 ORCHID<br />
PHARMACEUTICALS SA<br />
(PTY) LTD<br />
43/2.2/0248 AUROBINDO PHARMA<br />
(PTY) LTD<br />
BISOPROLOL<br />
HYDROCHLORO-<br />
THIAZIDE ZYDUS<br />
2,5/6,25 TABLETS<br />
BISOPROLOL<br />
HYDROCHLORO-<br />
THIAZIDE ZYDUS<br />
5/6,25 TABLETS<br />
BISOPROLOL<br />
HYDROCHLORO-<br />
THIAZIDE ZYDUS<br />
10/6,25 TABLETS<br />
ORCHID<br />
CEFPODOXIME<br />
40 mg/5 ml<br />
AURO ZOLPIDEM<br />
10 mg<br />
Each vial contains:<br />
AZITHROMYCIN<br />
MONOHYDRATE equivalent to<br />
AZITHROMYCIN 500,0 mg<br />
Each tablet contains:<br />
ANASTROZOLE 1,0 mg<br />
Each tablet contains:<br />
BISOPROLOL FUMARATE<br />
2,5 mg<br />
HYDROCHLOROTHIAZIDE<br />
6,25 mg<br />
Each tablet contains:<br />
BISOPROLOL FUMARATE<br />
5,0 mg<br />
HYDROCHLOROTHIAZIDE<br />
6,25 mg<br />
Each tablet contains:<br />
BISOPROLOL FUMARATE<br />
10,0 mg<br />
HYDROCHLOROTHIAZIDE<br />
6,25 mg<br />
Each 5,0 ml suspension<br />
contains:<br />
CEFPODOXIME PROXETIL<br />
equivalent to<br />
CEFPODOXIME 40,0 mg<br />
Each tablet contains:<br />
ZOLPIDEM TARTRATE<br />
10,0 mg<br />
<strong>12.68</strong>_<strong>Notification</strong>_<strong>of</strong>_<strong>Registration</strong>_<strong>Sep12</strong>_<strong>v1</strong> Oct 2012 Page 4 <strong>of</strong> 10
Administration <strong>Notification</strong> <strong>of</strong> <strong>Registration</strong> <strong>of</strong> <strong>Medicines</strong><br />
Application<br />
Number<br />
Holder <strong>of</strong> Certificate <strong>of</strong><br />
<strong>Registration</strong><br />
43/2.2/0258 AUROBINDO PHARMA<br />
(PTY) LTD<br />
43/1.2/0273 BE-TABS<br />
PHARMACEUTICALS<br />
(PTY) LTD<br />
43/1.2/0274 RANBAXY (SA) (PTY)<br />
LTD<br />
43/1.2/0275 BE-TABS<br />
PHARMACEUTICALS<br />
(PTY) LTD<br />
43/1.2/0276 RANBAXY (SA) (PTY)<br />
LTD<br />
43/1.2/0312 PHARMA DYNAMICS<br />
(PTY) LTD<br />
43/1.2/0313 PHARMA DYNAMICS<br />
(PTY) LTD<br />
43/1.2/0314 PHARMA DYNAMICS<br />
(PTY) LTD<br />
43/1.2/0315 PHARMA DYNAMICS<br />
(PTY) LTD<br />
Proprietary Name Active Compound<br />
AURO ZOLPIDEM 5 mg Each tablet contains:<br />
ZOLPIDEM TARTRATE 5,0 mg<br />
BESIT 10 Each tablet contains:<br />
ESCITALOPRAM OXALATE<br />
equivalent to<br />
ESCITALOPRAM 10,0 mg<br />
EPRALIFE 10 Each tablet contains:<br />
ESCITALOPRAM OXALATE<br />
equivalent to<br />
ESCITALOPRAM 10,0 mg<br />
BESIT 20 Each tablet contains:<br />
ESCITALOPRAM OXALATE<br />
equivalent to<br />
ESCITALOPRAM 20,0 mg<br />
EPRALIFE 20 Each tablet contains:<br />
ESCITALOPRAM OXALATE<br />
equivalent to<br />
ESCITALOPRAM 20,0 mg<br />
DYNA ESCITALOPRAM<br />
5 mg<br />
DYNA ESCITALOPRAM<br />
10 mg<br />
DYNA ESCITALOPRAM<br />
15 mg<br />
DYNA ESCITALOPRAM<br />
20 mg<br />
Each tablet contains:<br />
ESCITALOPRAM OXALATE<br />
equivalent to<br />
ESCITALOPRAM 5,0 mg<br />
Each tablet contains:<br />
ESCITALOPRAM OXALATE<br />
equivalent to<br />
ESCITALOPRAM 10,0 mg<br />
Each tablet contains:<br />
ESCITALOPRAM OXALATE<br />
equivalent to<br />
ESCITALOPRAM 15,0 mg<br />
Each tablet contains:<br />
ESCITALOPRAM OXALATE<br />
equivalent to<br />
ESCITALOPRAM 20,0 mg<br />
43/20.1.8/0388 PHARMACARE LIMITED VAVIREX 500 Each tablet contains:<br />
VALACICLOVIR<br />
HYDROCHLORIDE equivalent<br />
to VALACICLOVIR 500,0 mg<br />
43/11.4.3/0483 SANDOZ SA (PTY) LTD TOPRALOC 20 Each tablet contains:<br />
PANTOPRAZOLE SODIUM<br />
SESQUIHYDRATE equivalent to<br />
PANTOPRAZOLE 20,0 mg<br />
43/11.4.3/0484 SANDOZ SA (PTY) LTD TOPRALOC 40 Each tablet contains:<br />
PANTOPRAZOLE SODIUM<br />
SESQUIHYDRATE equivalent to<br />
PANTOPRAZOLE 40,0 mg<br />
43/11.4.3/0485 SANDOZ SA (PTY) LTD HEXAL<br />
PANTOPRAZOLE 20<br />
43/11.4.3/0486 SANDOZ SA (PTY) LTD HEXAL<br />
PANTOPRAZOLE 40<br />
Each tablet contains:<br />
PANTOPRAZOLE SODIUM<br />
SESQUIHYDRATE equivalent to<br />
PANTOPRAZOLE 20,0 mg<br />
Each tablet contains:<br />
PANTOPRAZOLE SODIUM<br />
SESQUIHYDRATE equivalent to<br />
PANTOPRAZOLE 40,0 mg<br />
<strong>12.68</strong>_<strong>Notification</strong>_<strong>of</strong>_<strong>Registration</strong>_<strong>Sep12</strong>_<strong>v1</strong> Oct 2012 Page 5 <strong>of</strong> 10
Administration <strong>Notification</strong> <strong>of</strong> <strong>Registration</strong> <strong>of</strong> <strong>Medicines</strong><br />
Application<br />
Number<br />
Holder <strong>of</strong> Certificate <strong>of</strong><br />
<strong>Registration</strong><br />
43/7.1/0558 BIOTECH<br />
LABORATORIES (PTY)<br />
LTD<br />
43/7.1/0559 BIOTECH<br />
LABORATORIES (PTY)<br />
LTD<br />
Proprietary Name Active Compound<br />
BIO NIFEDIPINE XL 30 Each tablet contains:<br />
NIFEDIPINE 30,0 mg<br />
BIO NIFEDIPINE XL 60 Each tablet contains:<br />
NIFEDIPINE 60,0 mg<br />
43/2.6.5/0593 PHARMAPLAN (PTY) LTD ZOQIT 25 Each tablet contains:<br />
QUETIAPINE FUMARATE<br />
equivalent to<br />
QUETIAPINE 25,0 mg<br />
43/2.6.5/0594 PHARMAPLAN (PTY) LTD ZOQIT 100 Each tablet contains:<br />
QUETIAPINE FUMARATE<br />
equivalent to<br />
QUETIAPINE 100,0 mg<br />
43/2.6.5/0595 PHARMAPLAN (PTY) LTD ZOQIT 200 Each tablet contains:<br />
QUETIAPINE FUMARATE<br />
equivalent to<br />
QUETIAPINE 200,0 mg<br />
43/2.6.5/0596 PHARMAPLAN (PTY) LTD ZOQIT 300 Each tablet contains:<br />
QUETIAPINE FUMARATE<br />
equivalent to<br />
QUETIAPINE 300,0 mg<br />
43/13.1/0673 BARRS<br />
PHARMACEUTICAL<br />
INDUSTRIES cc<br />
43/13.1/0674 BARRS<br />
PHARMACEUTICAL<br />
INDUSTRIES cc<br />
43/2.2/0819 DR REDDY’S<br />
LABORATORIES (PTY)<br />
LTD<br />
43/5.7.1/0912 SCHERING-PLOUGH<br />
(PTY) LTD<br />
43/20.1.1/1087 CIPLA MEDPRO (PTY)<br />
LTD<br />
43/20.1.1/1088 CIPLA LIFE SCIENCES<br />
(PTY) LTD<br />
STERISCRUB Each 100,0 ml solution contains:<br />
CHLORHEXIDINE<br />
GLUCONATE 20 % SOLUTION<br />
20,0 ml<br />
STERISOL Each 100,0 ml solution contains:<br />
CHLORHEXIDINE<br />
GLUCONATE 20 % SOLUTION<br />
2,50 ml<br />
DRL ZOLPIDEM 10 Each tablet contains:<br />
ZOLPIDEM TARTRATE<br />
10,0 mg<br />
DESELEX D-12 Each tablet contains:<br />
DESLORATADINE 2,5 mg<br />
PSEUDOEPHEDRINE<br />
SULPHATE 120,0 mg<br />
MEDPRO VANCOMAX<br />
500<br />
Each vial contains:<br />
VANCOMYCIN HCl equivalent<br />
to VANCOMYCIN 500,0 mg<br />
CIPLA VANCOMYCIN Each vial contains:<br />
VANCOMYCIN HCl equivalent<br />
to VANCOMYCIN 500,0 mg<br />
43/2.5/1142 PHARMAPLAN (PTY) LTD TOPIROL 50 Each tablet contains:<br />
TOPIRAMATE 50,0 mg<br />
43/26/1171 CIPLA MEDPRO (PTY)<br />
LTD<br />
43/26/1172 CIPLA MEDPRO (PTY)<br />
LTD<br />
ONCOGEM 200 Each vial contains:<br />
GEMCITABINE<br />
HYDROCHLORIDE equivalent<br />
to GEMCITABINE 200,0 mg<br />
ONCOGEM 1 000 Each vial contains:<br />
GEMCITABINE<br />
HYDROCHLORIDE equivalent<br />
to GEMCITABINE 1 000,0 mg<br />
<strong>12.68</strong>_<strong>Notification</strong>_<strong>of</strong>_<strong>Registration</strong>_<strong>Sep12</strong>_<strong>v1</strong> Oct 2012 Page 6 <strong>of</strong> 10
Administration <strong>Notification</strong> <strong>of</strong> <strong>Registration</strong> <strong>of</strong> <strong>Medicines</strong><br />
Application<br />
Number<br />
Holder <strong>of</strong> Certificate <strong>of</strong><br />
<strong>Registration</strong><br />
Proprietary Name Active Compound<br />
44/2.9/0113 PHARMAPLAN (PTY) LTD DEPODUR 10 Each vial contains:<br />
MORPHINE SULPHATE<br />
PENTAHYDRATE 10,0 mg<br />
44/21.12/0281 CIPLA MEDPRO (PTY)<br />
LTD<br />
44/21.12/0282 CIPLE LIFE SCIENCES<br />
(PTY) LTD<br />
MEDPRO LETROZOLE<br />
2,5<br />
Each tablet contains:<br />
LETROZOLE 2,5 mg<br />
CIPLA LETROZOLE 2,5 Each tablet contains:<br />
LETROZOLE 2,5 mg<br />
44/3.1/0331 NYCOMED (PTY) LTD XEFO RAPID Each tablet contains:<br />
LORNOXICAM 8,0 mg<br />
44/26/0380 ACCORD HEALTHCARE<br />
(PTY) LTD<br />
44/26/0381 ACCORD HEALTHCARE<br />
(PTY) LTD<br />
44/2.6.5/0447 AKACIA HEALTHCARE<br />
(PTY) LTD<br />
44/2.6.5/0448 AKACIA HEALTHCARE<br />
(PTY) LTD<br />
44/2.6.5/0449 AKACIA HEALTHCARE<br />
(PTY) LTD<br />
44/2.6.5/0450 AKACIA HEALTHCARE<br />
(PTY) LTD<br />
44/2.6.5/0451 AKACIA HEALTHCARE<br />
(PTY) LTD<br />
44/2.6.5/0452 AKACIA HEALTHCARE<br />
(PTY) LTD<br />
44/2.6.5/0453 AKACIA HEALTHCARE<br />
(PTY) LTD<br />
44/2.6.5/0454 AKACIA HEALTHCARE<br />
(PTY) LTD<br />
44/7.1.3/0477 RANBAXY (SA) (PTY)<br />
LTD<br />
44/7.1.3/0478 RANBAXY (SA) (PTY)<br />
LTD<br />
44/7.1.3/0479 RANBAXY (SA) (PTY)<br />
LTD<br />
44/7.1.3/0482 RANBAXY (SA) (PTY)<br />
LTD<br />
44/7.1.3/0483 RANBAXY (SA) (PTY)<br />
LTD<br />
INTAS OXALIPLATIN<br />
50<br />
ACCORD<br />
OXALIPLATIN 50<br />
Each vial contains:<br />
OXALIPLATIN 50,0 mg<br />
Each vial contains:<br />
OXALIPLATIN 50,0 mg<br />
ZYLENA 5 Each tablet contains:<br />
OLANZAPINE 5,0 mg<br />
ZYLENA 10 Each tablet contains:<br />
OLANZAPINE 10,0 mg<br />
ZYLENA 15 Each tablet contains:<br />
OLANZAPINE 15,0 mg<br />
ZYLENA 20 Each tablet contains:<br />
OLANZAPINE 20,0 mg<br />
ZYLENA ODT 5 Each tablet contains:<br />
OLANZAPINE 5,0 mg<br />
ZYLENA ODT 10 Each tablet contains:<br />
OLANZAPINE 10,0 mg<br />
ZYLENA ODT 15 Each tablet contains:<br />
OLANZAPINE 15,0 mg<br />
ZYLENA ODT 20 Each tablet contains:<br />
OLANZAPINE 20,0 mg<br />
DIOLO CO PLUS<br />
160/25<br />
Each tablet contains:<br />
VALSARTAN 160,0 mg<br />
HYDROCHLOROTHIAZIDE<br />
25,0 mg<br />
DIOLO CO 80/12,5 Each tablet contains:<br />
VALSARTAN 80,0 mg<br />
HYDROCHLOROTHIAZIDE<br />
12,5 mg<br />
DIOLO CO 160/12,5 Each tablet contains:<br />
VALSARTAN 160,0 mg<br />
HYDROCHLOROTHIAZIDE<br />
12,5 mg<br />
VALSARTAN RAN CO<br />
160/25<br />
VALSARTAN RAN CO<br />
80/12,5<br />
Each tablet contains:<br />
VALSARTAN 160,0 mg<br />
HYDROCHLOROTHIAZIDE<br />
25,0 mg<br />
Each tablet contains:<br />
VALSARTAN 80,0 mg<br />
HYDROCHLOROTHIAZIDE<br />
12,5 mg<br />
<strong>12.68</strong>_<strong>Notification</strong>_<strong>of</strong>_<strong>Registration</strong>_<strong>Sep12</strong>_<strong>v1</strong> Oct 2012 Page 7 <strong>of</strong> 10
Administration <strong>Notification</strong> <strong>of</strong> <strong>Registration</strong> <strong>of</strong> <strong>Medicines</strong><br />
Application<br />
Number<br />
Holder <strong>of</strong> Certificate <strong>of</strong><br />
<strong>Registration</strong><br />
44/7.1.3/0484 RANBAXY (SA) (PTY)<br />
LTD<br />
44/7.1.3/0560 RANBAXY (SA) (PTY)<br />
LTD<br />
44/7.1.3/0561 RANBAXY (SA) (PTY)<br />
LTD<br />
44/7.1.3/0562 RANBAXY (SA) (PTY)<br />
LTD<br />
44/7.1.3/0563 RANBAXY (SA) (PTY)<br />
LTD<br />
44/7.1.3/0564 RANBAXY (SA) (PTY)<br />
LTD<br />
44/7.1.3/0565 RANBAXY (SA) (PTY)<br />
LTD<br />
Proprietary Name Active Compound<br />
VALSARTAN RAN CO<br />
160/12,5<br />
Each tablet contains:<br />
VALSARTAN 160,0 mg<br />
HYDROCHLOROTHIAZIDE<br />
12,5 mg<br />
ZIRINAK 2,5/6,25 Each tablet contains:<br />
BISOPROLOL FUMARATE<br />
2,5 mg<br />
HYDROCHLOROTHIAZIDE<br />
6,25 mg<br />
ZIRINAK 5/6,25 Each tablet contains:<br />
BISOPROLOL FUMARATE<br />
5,0 mg<br />
HYDROCHLOROTHIAZIDE<br />
6,25 mg<br />
ZIRINAK 10/6,25 Each tablet contains:<br />
BISOPROLOL FUMARATE<br />
10,0 mg<br />
HYDROCHLOROTHIAZIDE<br />
6,25 mg<br />
RAN BISOPROLOL CO<br />
2,5/6,25<br />
RAN BISOPROLOL CO<br />
5/6,25<br />
RAN BISOPROLOL CO<br />
10/6,25<br />
44/11.4.3/0595 SANDOZ SA (PTY) LTD SANDOZ<br />
RABEPRAZOLE 10<br />
44/11.4.3/0596 SANDOZ SA (PTY) LTD SANDOZ<br />
RABEPRAZOLE 20<br />
Each tablet contains:<br />
BISOPROLOL FUMARATE<br />
2,5 mg<br />
HYDROCHLOROTHIAZIDE<br />
6,25 mg<br />
Each tablet contains:<br />
BISOPROLOL FUMARATE<br />
5,0 mg<br />
HYDROCHLOROTHIAZIDE<br />
6,25 mg<br />
Each tablet contains:<br />
BISOPROLOL FUMARATE<br />
10,0 mg<br />
HYDROCHLOROTHIAZIDE<br />
6,25 mg<br />
Each tablet contains:<br />
RABEPRAZOLE SODIUM<br />
10,0 mg<br />
Each tablet contains:<br />
RABEPRAZOLE SODIUM<br />
20,0 mg<br />
44/11.4.3/0597 SANDOZ SA (PTY) LTD RABEMED 10 Each tablet contains:<br />
RABEPRAZOLE SODIUM<br />
10,0 mg<br />
44/11.4.3/0598 SANDOZ SA (PTY) LTD RABEMED 20 Each tablet contains:<br />
RABEPRAZOLE SODIUM<br />
20,0 mg<br />
44/21.2/0794 ALKEM LABORATORIES<br />
(PTY) LTD<br />
44/21.2/0795 ALKEM LABORATORIES<br />
(PTY) LTD<br />
44/7.1.3/0906 BRIMPHARM SA (PTY)<br />
LTD<br />
METFORM ALKEM 500 Each tablet contains:<br />
METFORMIN<br />
HYDROCHLORIDE 500,0 mg<br />
METFORM ALKEM 850 Each tablet contains:<br />
METFORMIN<br />
HYDROCHLORIDE 850,0 mg<br />
IRTANEL 75 Each tablet contains:<br />
IRBESARTAN 75,0 mg<br />
<strong>12.68</strong>_<strong>Notification</strong>_<strong>of</strong>_<strong>Registration</strong>_<strong>Sep12</strong>_<strong>v1</strong> Oct 2012 Page 8 <strong>of</strong> 10
Administration <strong>Notification</strong> <strong>of</strong> <strong>Registration</strong> <strong>of</strong> <strong>Medicines</strong><br />
Application<br />
Number<br />
Holder <strong>of</strong> Certificate <strong>of</strong><br />
<strong>Registration</strong><br />
44/7.1.3/0907 BRIMPHARM SA (PTY)<br />
LTD<br />
44/7.1.3/0908 BRIMPHARM SA (PTY)<br />
LTD<br />
44/7.1.3/0909 BRIMPHARM SA (PTY)<br />
LTD<br />
44/7.1.3/0910 BRIMPHARM SA (PTY)<br />
LTD<br />
44/7.1.3/0911 BRIMPHARM SA (PTY)<br />
LTD<br />
44/20.2.2/0932 JANSSEN<br />
PHARMACEUTICA (PTY)<br />
LTD<br />
44/1.2/1005 ZYDUS HEALTHCARE SA<br />
(PTY) LTD<br />
44/1.2/1006 ZYDUS HEALTHCARE SA<br />
(PTY) LTD<br />
44/1.2/1007 ZYDUS HEALTHCARE SA<br />
(PTY) LTD<br />
44/1.2/1008 ZYDUS HEALTHCARE SA<br />
(PTY) LTD<br />
44/20.2.8/1026 MACLEODS<br />
PHARMACEUTICALS SA<br />
(PTY) LTD<br />
44/20.2.8/1027 MACLEODS<br />
PHARMACEUTICALS SA<br />
(PTY) LTD<br />
44/20.2.8/1028 MACLEODS<br />
PHARMACEUTICALS SA<br />
(PTY) LTD<br />
Proprietary Name Active Compound<br />
IRTANEL 150 Each tablet contains:<br />
IRBESARTAN 150,0 mg<br />
IRTANEL 300 Each tablet contains:<br />
IRBESARTAN 300,0 mg<br />
IRBESARTAN<br />
BRIMPHARM 75<br />
IRBESARTAN<br />
BRIMPHARM 150<br />
IRBESARTAN<br />
BRIMPHARM 300<br />
Each tablet contains:<br />
IRBESARTAN 75,0 mg<br />
Each tablet contains:<br />
IRBESARTAN 150,0 mg<br />
Each tablet contains:<br />
IRBESARTAN 300,0 mg<br />
DAK-O-GEL Each 1,0 g gel contains:<br />
MICONAZOLE 20,0 mg<br />
MIRTAZAPINE ZYDUS<br />
15 mg<br />
MIRTAZAPINE ZYDUS<br />
30 mg<br />
Each tablet contains:<br />
MIRTAZAPINE 15,0 mg<br />
Each tablet contains:<br />
MIRTAZAPINE 30,0 mg<br />
MIRADEP 15 mg Each tablet contains:<br />
MIRTAZAPINE 15,0 mg<br />
MIRADEP 30 mg Each tablet contains:<br />
MIRTAZAPINE 30,0 mg<br />
ROSTAV 15 Each capsule contains:<br />
STAVUDINE 15,0 mg<br />
ROSTAV 20 Each capsule contains:<br />
STAVUDINE 20,0 mg<br />
ROSTAV 30 Each capsule contains:<br />
STAVUDINE 30,0 mg<br />
44/20.2.8/1051 PHARMACARE LIMITED TENARENZ Each tablet contains:<br />
TENOFOVIR DISOPROXIL<br />
FUMARATE 300,0 mg<br />
LAMIVUDINE 300,0 mg<br />
EFAVIRENZ 600,0 mg<br />
44/2.9/1087 PHARMAPLAN (PTY) LTD DEPODUR 15 Each vial contains:<br />
MORPHINE SULPHATE<br />
PENTAHYDRATE 15,0 mg<br />
44/2.9/1088 PHARMAPLAN (PTY) LTD DEPODUR 20 Each vial contains:<br />
MORPHINE SULPHATE<br />
PENTAHYDRATE 20,0 mg<br />
45/5.7.2/0067 TEVA<br />
PHARMACEUTICALS<br />
(PTY) LTD<br />
45/5.7.2/0068 TEVA<br />
PHARMACEUTICALS<br />
(PTY) LTD<br />
45/20.2.8/0108 CIPLA MEDPRO (PTY)<br />
LTD<br />
GRANISETRON TEVA<br />
1<br />
GRANISETRON TEVA<br />
3<br />
CIPLA LAMIVUDINE &<br />
TENOFOVIR 300/300<br />
Each 1,0 ml solution contains:<br />
GRANISETRON<br />
HYDROCHLORIDE equivalent<br />
to GRANISETRON 1,0 mg<br />
Each 3,0 ml solution contains:<br />
GRANISETRON<br />
HYDROCHLORIDE equivalent<br />
to GRANISETRON 3,0 mg<br />
Each tablet contains:<br />
TENOFOVIR DISOPROXIL<br />
FUMARATE 300,0 mg<br />
LAMIVUDINE 300,0 mg<br />
<strong>12.68</strong>_<strong>Notification</strong>_<strong>of</strong>_<strong>Registration</strong>_<strong>Sep12</strong>_<strong>v1</strong> Oct 2012 Page 9 <strong>of</strong> 10
Administration <strong>Notification</strong> <strong>of</strong> <strong>Registration</strong> <strong>of</strong> <strong>Medicines</strong><br />
Application<br />
Number<br />
Holder <strong>of</strong> Certificate <strong>of</strong><br />
<strong>Registration</strong><br />
45/20.2.8/0109 CIPLA LIFE SCIENCES<br />
(PTY) LTD<br />
45/26/0145 CIPLA MEDPRO (PTY)<br />
LTD<br />
45/26/0146 CIPLA LIFE SCIENCES<br />
(PTY) LTD<br />
45/8.2/0162 INGELHEIM<br />
PHARMACEUTICALS<br />
(PTY) LTD<br />
45/32.2/0540 BAROQUE<br />
PHARMACEUTICALS<br />
(PTY) LTD<br />
45/10.2.2/0744 CAMOX<br />
PHARMACEUTICALS<br />
(PTY) LTD<br />
45/10.2.2/0745 CAMOX<br />
PHARMACEUTICALS<br />
(PTY) LTD<br />
45/20.2.8/0887 MACLEODS<br />
PHARMACEUTICALS SA<br />
(PTY) LTD<br />
Proprietary Name Active Compound<br />
MEDPRO LAMIVUDINE<br />
& TENOFOVIR 300/300<br />
Each tablet contains:<br />
TENOFOVIR DISOPROXIL<br />
FUMARATE 300,0 mg<br />
LAMIVUDINE 300,0 mg<br />
PURIDA Each vial contains:<br />
FLUDARABINE 50,0 mg<br />
CIPLA FLUDARABINE Each vial contains:<br />
FLUDARABINE 50,0 mg<br />
PRADAXA 150 Each capsule contains:<br />
DABIGATRAN ETEXILATE<br />
MESILATE equivalent to<br />
DABIGATRAN ETEXILATE<br />
150,0 mg<br />
XIENCE XPEDITION Each stent contains:<br />
EVEROLIMUS 100,0 µg/cm 2<br />
BELAIR 4 mg Each chewable tablet contains:<br />
MONTELUKAST SODIUM<br />
equivalent to<br />
MONTELUKAST 4,0 mg<br />
BELAIR 5 mg Each chewable tablet contains:<br />
MONTELUKAST SODIUM<br />
equivalent to<br />
MONTELUKAST 5,0 mg<br />
MACLEODS<br />
STAVUDINE 30 mg<br />
45/20.2.8/0926 MYLAN (PTY) LTD MYLAN<br />
EMTRICITABINE<br />
200 mg<br />
Each tablet contains:<br />
STAVUDINE 30,0 mg<br />
Each capsule contains:<br />
EMTRICITABINE 200,0 mg<br />
46/20.2.8/0006 MYLAN (PTY) LTD KAVIMUN PAED Each tablet contains:<br />
ABACAVIR SULPHATE<br />
equivalent to<br />
ABACAVIR 60,0 mg<br />
46/20.2.8/0008 MYLAN (PTY) LTD ZIDOMAT 100 mg Each tablet contains:<br />
ZIDOVUDINE 100,0 mg<br />
46/20.2.8/0009 MYLAN (PTY) LTD ZIDOMAT 300 mg Each tablet contains:<br />
ZIDOVUDINE 300,0 mg<br />
46/34/0023 NOVARTIS SA (PTY) LTD GILENYA 0,5 mg Each capsule contains:<br />
FINGOLIMOD<br />
HYDROCHLORIDE 0,5 mg<br />
MS MANDISA HELA<br />
REGISTRAR OF MEDICINES<br />
<strong>12.68</strong>_<strong>Notification</strong>_<strong>of</strong>_<strong>Registration</strong>_<strong>Sep12</strong>_<strong>v1</strong> Oct 2012 Page 10 <strong>of</strong> 10