sudoku Kinetics
sudoku Kinetics
sudoku Kinetics
You also want an ePaper? Increase the reach of your titles
YUMPU automatically turns print PDFs into web optimized ePapers that Google loves.
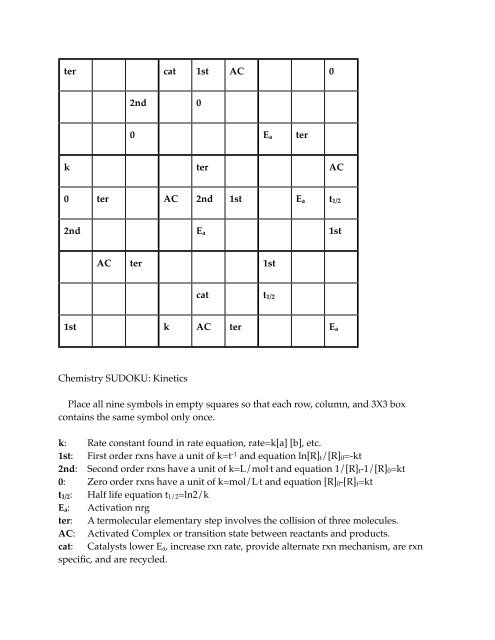
ter cat 1st AC 0<br />
2nd 0<br />
0 E a ter<br />
k ter AC<br />
0 ter AC 2nd 1st E a t 1/2<br />
2nd E a 1st<br />
AC ter 1st<br />
cat t 1/2<br />
1st k AC ter E a<br />
Chemistry SUDOKU: <strong>Kinetics</strong><br />
Place all nine symbols in empty squares so that each row, column, and 3X3 box<br />
contains the same symbol only once.<br />
k: Rate constant found in rate equation, rate=k[a] [b], etc.<br />
1st: First order rxns have a unit of k=t -1 and equation ln[R] t /[R] 0 =-kt<br />
2nd: Second order rxns have a unit of k=L/mol . t and equation 1/[R] t -1/[R] 0 =kt<br />
0: Zero order rxns have a unit of k=mol/L . t and equation [R] 0 -[R] t =kt<br />
t 1/2 : Half life equation t 1/2 =ln2/k<br />
E a : Activation nrg<br />
ter: A termolecular elementary step involves the collision of three molecules.<br />
AC: Activated Complex or transition state between reactants and products.<br />
cat: Catalysts lower E a , increase rxn rate, provide alternate rxn mechanism, are rxn<br />
specific, and are recycled.