Tabel 7.pdf - Ordbogen.com
Tabel 7.pdf - Ordbogen.com
Tabel 7.pdf - Ordbogen.com
You also want an ePaper? Increase the reach of your titles
YUMPU automatically turns print PDFs into web optimized ePapers that Google loves.
<strong>Tabel</strong> 7<br />
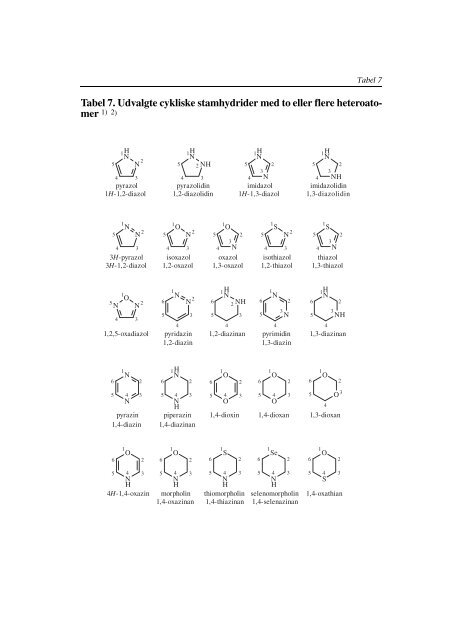
<strong>Tabel</strong> 7. Udvalgte cykliske stamhydrider med to eller flere heteroatomer<br />
1) 2)<br />
<strong>Tabel</strong> 7<br />
N<br />
N<br />
NH<br />
N<br />
N<br />
N<br />
N<br />
N<br />
N<br />
N<br />
N<br />
O<br />
N<br />
O<br />
N<br />
S<br />
N<br />
S<br />
pyrazol<br />
1H-1,2-diazol<br />
pyrazolidin<br />
1,2-diazolidin<br />
imidazol<br />
1H-1,3-diazol<br />
imidazolidin<br />
1,3-diazolidin<br />
H H H<br />
H<br />
1<br />
2<br />
3<br />
4<br />
5<br />
1<br />
2<br />
3<br />
4<br />
5<br />
1<br />
2<br />
3<br />
4<br />
5<br />
1<br />
2<br />
3<br />
4<br />
5<br />
3H-pyrazol<br />
3H-1,2-diazol<br />
isoxazol<br />
1,2-oxazol<br />
1,3-oxazol<br />
isothiazol<br />
1,2-thiazol<br />
thiazol<br />
1<br />
2<br />
3<br />
4<br />
5<br />
1<br />
2<br />
3<br />
4<br />
5<br />
1<br />
2<br />
3<br />
4<br />
5<br />
1<br />
2<br />
3<br />
4<br />
5<br />
1<br />
2<br />
3<br />
4<br />
5<br />
N<br />
N<br />
NH<br />
N<br />
N<br />
N<br />
NH<br />
N<br />
N<br />
O<br />
N<br />
N<br />
N<br />
O<br />
O<br />
O<br />
O<br />
O<br />
O<br />
N<br />
O<br />
N<br />
S<br />
N<br />
Se<br />
S<br />
O<br />
H<br />
1<br />
2<br />
3<br />
4<br />
5<br />
1<br />
2<br />
3<br />
4<br />
5<br />
6<br />
1<br />
2<br />
3<br />
4<br />
5<br />
6<br />
1<br />
2<br />
3<br />
4<br />
5<br />
6<br />
1<br />
2<br />
3<br />
4<br />
5<br />
6<br />
1,2,5-oxadiazol pyridazin 1,2-diazinan pyrimidin 1,3-diazinan<br />
1,3-diazin<br />
1,2-diazin<br />
N<br />
N<br />
1<br />
2<br />
3<br />
4<br />
5<br />
6<br />
H<br />
H<br />
H<br />
H<br />
1<br />
2<br />
3<br />
4<br />
5<br />
6<br />
1<br />
2<br />
3<br />
4<br />
5<br />
6<br />
1<br />
2<br />
3<br />
4<br />
5<br />
6<br />
1<br />
2<br />
3<br />
4<br />
5<br />
6<br />
pyrazin<br />
1,4-diazin<br />
piperazin<br />
1,4-diazinan<br />
1,4-dioxin 1,4-dioxan 1,3-dioxan<br />
N<br />
O<br />
H H H H<br />
1<br />
2<br />
3<br />
4<br />
5<br />
6<br />
1<br />
2<br />
3<br />
4<br />
5<br />
6<br />
1<br />
2<br />
3<br />
4<br />
5<br />
6<br />
1<br />
2<br />
3<br />
4<br />
5<br />
6<br />
1<br />
2<br />
3<br />
4<br />
5<br />
6<br />
4H-1,4-oxazin<br />
morpholin<br />
1,4-oxazinan<br />
thiomorpholin<br />
1,4-thiazinan<br />
selenomorpholin<br />
1,4-selenazinan<br />
1,4-oxathian<br />
oxazol<br />
1,3-thiazol
<strong>Tabel</strong> 7<br />
1<br />
6 O 2<br />
HB BH<br />
5 3<br />
O O<br />
B<br />
4<br />
H<br />
boroxin<br />
1,3,5,2,4,6-trioxatriborinan<br />
cyclotriboroxan<br />
1<br />
6 S 2<br />
HB BH<br />
5 3<br />
S 4 S<br />
B<br />
H<br />
borthiin<br />
1,3,5,2,4,6-trithiatriborinan<br />
cyclotriborathian<br />
H<br />
6 N 2<br />
HB 1 BH<br />
5 3<br />
HN NH<br />
B<br />
4<br />
H<br />
borazin<br />
1,3,5,2,4,6-triazatriborinan<br />
cyclotriborazan<br />
6<br />
5<br />
7<br />
1<br />
N<br />
2<br />
N<br />
5<br />
4<br />
3<br />
6<br />
indazol (her 3H-) benzimidazol (her 1H-) benzotriazol (her 1H-)<br />
H<br />
N<br />
N<br />
H<br />
N<br />
7 7<br />
1<br />
1<br />
6<br />
2<br />
2<br />
3<br />
5<br />
3<br />
4<br />
4<br />
N<br />
N<br />
7<br />
6<br />
8<br />
1<br />
2<br />
3<br />
5 4<br />
N<br />
N<br />
N<br />
7<br />
8<br />
cinnolin quinazolin quinoxalin<br />
1<br />
6<br />
3<br />
5 4<br />
N<br />
8<br />
2<br />
7<br />
6<br />
5<br />
1<br />
N<br />
N<br />
2<br />
4 3<br />
7<br />
6<br />
8 1<br />
2<br />
3<br />
N<br />
N<br />
5 4<br />
5 4<br />
7<br />
6<br />
8<br />
N<br />
1<br />
N<br />
2<br />
7<br />
3<br />
6<br />
phthalazin naphthyridin (her 1,8-) 3) naphthyridin (her 1,5-) 3)<br />
8<br />
5<br />
N<br />
1<br />
N<br />
4<br />
2<br />
3<br />
1<br />
2<br />
N<br />
N<br />
H<br />
N<br />
6 6<br />
8 1<br />
5 7<br />
7<br />
N<br />
N N<br />
1 5<br />
N<br />
7<br />
8<br />
8<br />
3 9<br />
2<br />
3<br />
3 9<br />
6 5<br />
4 N<br />
N<br />
4 N<br />
N<br />
4<br />
purin (her 7 H-) 4) purin (her 2 H-) 4) pteridin<br />
N<br />
2
<strong>Tabel</strong> 7<br />
9<br />
HN<br />
1<br />
2<br />
3<br />
N<br />
4<br />
9<br />
10<br />
N<br />
1<br />
N<br />
2<br />
3<br />
4<br />
8<br />
7<br />
5<br />
8<br />
5<br />
6<br />
7 6<br />
perimidin (her 1H-) phenanthrolin (her 1,10-) 3)<br />
9 1 9<br />
1<br />
9<br />
1<br />
O<br />
8 10<br />
2 8<br />
10<br />
2<br />
X<br />
8<br />
X<br />
10<br />
2<br />
7<br />
6<br />
5<br />
O<br />
4<br />
oxanthren<br />
X = S:<br />
dibenzo[1,4]dioxin 5) X = Se:<br />
X = Te:<br />
X = Hg:<br />
3<br />
7<br />
6<br />
5<br />
X<br />
4<br />
thianthren<br />
selenanthren<br />
telluranthren<br />
mercuranthren 6)<br />
3<br />
7<br />
6<br />
X<br />
X = N:<br />
X = P:<br />
X = As:<br />
X = SiH:<br />
X = B:<br />
5<br />
4<br />
3<br />
phenazin<br />
phosphanthren<br />
arsanthren<br />
silanthren<br />
boranthren<br />
8<br />
9 1<br />
9<br />
1<br />
X<br />
10<br />
2<br />
8<br />
X<br />
10<br />
2<br />
7<br />
6<br />
X = S:<br />
X = Se:<br />
X = Te:<br />
X = NH:<br />
X = PH:<br />
X = AsH:<br />
X = SbH:<br />
5<br />
3<br />
O<br />
4<br />
phenoxathiin<br />
phenoxaselenin<br />
phenoxatellurin<br />
phenoxazin (her 10H-)<br />
phenoxaphosphin 7)<br />
phenoxaphosphinin (her 10H-)<br />
phenoxarsin 7)<br />
phenoxarsinin (her 10H-)<br />
phenoxantimonin 7)<br />
phenoxastibinin (her 10H-)<br />
8<br />
7<br />
9<br />
X = Se, Z = NH:<br />
X = Se, Z = PH:<br />
X = Te, Z = NH:<br />
6<br />
X = Se:<br />
X = NH:<br />
X = PH:<br />
X = AsH:<br />
Z<br />
10<br />
5<br />
X<br />
7<br />
1<br />
4<br />
6<br />
S<br />
phenothiaselenin<br />
phenothiazin (her 10H-)<br />
phenothiaphosphinin (her 10H-)<br />
phenothiarsin 7)<br />
phenothiarsinin (her 10H-)<br />
2<br />
3<br />
phenoselenazin (her 10H-)<br />
phenoselenaphosphinin (her 10H-)<br />
phenotellurazin (her 10H-)<br />
5<br />
4<br />
3
<strong>Tabel</strong> 7<br />
8<br />
9<br />
X<br />
10<br />
1 9<br />
2<br />
8<br />
X<br />
10<br />
1<br />
2<br />
7<br />
6<br />
5<br />
N<br />
X = P: phenophosphazinin 8)<br />
X = As: phenarsazinin 8)<br />
X = Sb: phenazastibinin 8)<br />
4<br />
3<br />
7<br />
6<br />
X = As:<br />
X = Sb:<br />
5<br />
P<br />
phenophospharsinin 8)<br />
phenophosphastibinin 8)<br />
4<br />
3<br />
9<br />
8<br />
7<br />
Hg<br />
10<br />
5<br />
1<br />
2<br />
3<br />
2<br />
N<br />
N<br />
6<br />
H<br />
4<br />
H<br />
5H-phenomercurazin 9) azandiyldi-o-phenylenkviksølv 9)<br />
Hg<br />
1) De anførte navne er stamnavne ( 2.3), hvis ikke andet er bemærket. Se også tabel 14D: bilan, corrin, phthalocyanin,<br />
porphyrin, yohimban.<br />
2) Hvor samme navn dækker over flere tautomerer, er kun vist én eller to tautomerer og de tilhørende navne med indiceret<br />
hydrogen. Hvor samme navn anvendes om flere isomerer, der adskiller sig ved heteroatomers positioner, er kun vist én<br />
eller to isomerer og de tilhørende navne med eksplicitte heteroatomlokanter.<br />
3) Tilsvarende 1,6-; 1,7-; 2,6- og 2,7-naphthyridin og 1,7-; 1,8-; 1,9-; 2,7-; 2,8-; 2,9-; 3,7-; 3,8- og 4,7-phenanthrolin; alle<br />
med ét nitrogenatom i hver terminal ring. Nummereringen i phenanthrolinskelettet er forskellig fra nummereringen af<br />
den tilgrundliggende carbocykliske stamforbindelse, phenanthren (tabel 5).<br />
4) Ukonventionel nummerering.<br />
5) Fusionsnavn ( 2.3.5.2) ud fra Hantzsch-Widman-navnet 1,4-dioxin (se ovenfor i tabellen).<br />
6) Tidligere phenomercurin. Se også koordinationsnavnet foreslået i 3.9.2.2.<br />
7) Disse navne er foreslået i [3, s. 466-471] og i en tabel i [4, s. 170], men forladt i 1998 [8].<br />
8) Disse navne med gruppe 15-heteroatomaffikser er så vidt vides i overensstemmelse med 1998-reglerne [8]. Navnene<br />
phenophosphazinin og phenarsazinin er nævnt eksplicit, og det påpeges, at de bryder Hantzsch-Widman-rækkefølgen.<br />
9) Koordinationsnavnet kan ikke bruges som stamnavn, da der ikke er nogen oplagt nummerering.