CHARACTERIZATION OF ANTI-CORROSION TRIAZOLE FILM By ...
CHARACTERIZATION OF ANTI-CORROSION TRIAZOLE FILM By ...
CHARACTERIZATION OF ANTI-CORROSION TRIAZOLE FILM By ...
Create successful ePaper yourself
Turn your PDF publications into a flip-book with our unique Google optimized e-Paper software.
J.Sc. Tech ـــــــــــــــــــــــــــــــــــــــــــــــــــــــــــــــــــــــــــــــــــــــــــــــ ــــــــــــــــــــــــــــــVol.<br />
10(2) 2009<br />
area at the lower part which was exposed to corrosive medium i.e. Red sea<br />
water. The zinc electrode was polished with sand paper, washed in running<br />
tap water, rinsed in distilled water before exposure to sea water and sea<br />
water containing 0.01M MTRA inhibitor. The cyclic voltamograms were<br />
obtained using an electrochemical system Autolab (Netherlands) at<br />
corrosion potentials over a potential window between –2000 and 550 mV (vs<br />
SCE) at sweeping rate of 10 mV/s. A three compartment cell with zinc as<br />
working electrode, platinum foil and saturated calomel electrode as counter<br />
and reference electrodes respectively were used for CV studies The scanning<br />
electron micrographs of the polished zinc surface after exposure to sea<br />
water and sea water containing 0.01M inhibitor were taken using vacuum<br />
scanning electron microscope model JEOL JSM-5600 LV interfaced to a<br />
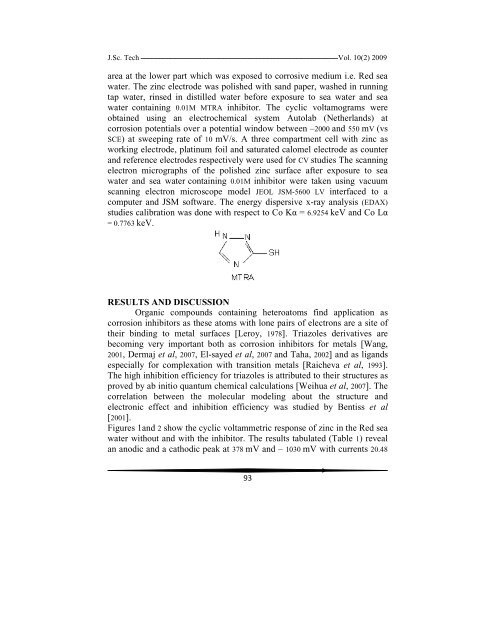
computer and JSM software. The energy dispersive x-ray analysis (EDAX)<br />
studies calibration was done with respect to Co Kα = 6.9254 keV and Co Lα<br />
= 0.7763 keV.<br />
RESULTS AND DISCUSSION<br />
Organic compounds containing heteroatoms find application as<br />
corrosion inhibitors as these atoms with lone pairs of electrons are a site of<br />
their binding to metal surfaces [Leroy, 1978]. Triazoles derivatives are<br />
becoming very important both as corrosion inhibitors for metals [Wang,<br />
2001, Dermaj et al, 2007, El-sayed et al, 2007 and Taha, 2002] and as ligands<br />
especially for complexation with transition metals [Raicheva et al, 1993].<br />
The high inhibition efficiency for triazoles is attributed to their structures as<br />
proved by ab initio quantum chemical calculations [Weihua et al, 2007]. The<br />
correlation between the molecular modeling about the structure and<br />
electronic effect and inhibition efficiency was studied by Bentiss et al<br />
[2001].<br />
Figures 1and 2 show the cyclic voltammetric response of zinc in the Red sea<br />
water without and with the inhibitor. The results tabulated (Table 1) reveal<br />
an anodic and a cathodic peak at 378 mV and – 1030 mV with currents 20.48<br />
93