The theory of membrane equilibria
The theory of membrane equilibria
The theory of membrane equilibria
You also want an ePaper? Increase the reach of your titles
YUMPU automatically turns print PDFs into web optimized ePapers that Google loves.
THEORY OF MEMBRANE EQUILIBRIA<br />
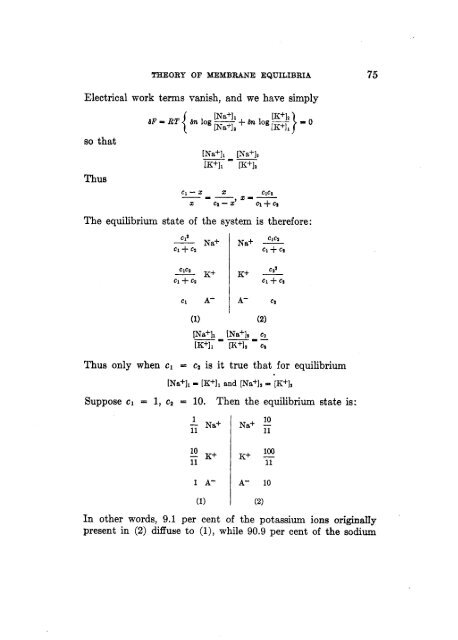
Electrical work terms vanish, and we have simply<br />
so that<br />
Thus<br />
c1-x 2 --- x=-<br />
1 - Na+<br />
11<br />
! K+<br />
11<br />
ClCZ<br />
x cz-x' Q+C<br />
<strong>The</strong> equilibrium state <strong>of</strong> the system<br />
1<br />
is therefore:<br />
c l9 ClCZ G~ Na+ Na+ -<br />
c1+ ct<br />
Thus only when c1 = c2 is it true that for equilibrium<br />
[Na+11 - [K+I, and [Na+l~ = [K+]z<br />
Suppose cl = 1, c2 = 10. <strong>The</strong>n the equilibrium state is:<br />
(1) A- 1<br />
A-<br />
10<br />
Na+ -<br />
11<br />
100<br />
K+ -<br />
11<br />
(2:<br />
In other words, 9.1 per cent <strong>of</strong> the potassium ions originaIIy<br />
present in (2) diffuse to (l), while 90.9 per cent <strong>of</strong> the sodium<br />
75